RNA interference/Citable Version: Difference between revisions
imported>David Tribe No edit summary |
imported>John Stephenson (remove unnecessary categories) |
||
| (375 intermediate revisions by 10 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | |||
[[Image:Biological_information_flow.gif|thumb|350px|In living cells, genes are expressed via a messenger RNA (mRNA) which participates in the synthesis of [[protein]]s. In turn, the catalytic activities of proteins decide many of the properties of a cell, that is, its outward [[phenotype]].]] | |||
'''RNA interference (RNAi)''' is a process that inhibits the flow of genetic information to protein synthesis, which normally proceeds from deoxyribonucleic acid ([[DNA]]) to messenger ribonucleic acid ([[mRNA]]) to proteins - a process known as [[gene expression]] (see figure, top right). mRNA, a single-stranded [[polymer]], is the key molecule whose sequence of [[nucleotide]]s transports the information encoded in DNA to regions of the cell where that DNA-genetic information is used for synthesizing [[protein]]s. Understanding that flow of information is necessary for understanding the RNAi mediated feedback loops that inhibit gene expression. | |||
RNAi | Specifically, RNAi is a mechanism in [[eukaryotic cells]] that is triggered when such cells are exposed to certain double-stranded ribonucleic acid (dsRNA) molecules. The process has been detected in many organisms, including animal, plant and protist cells. The distinguishing characteristic of RNAi is destruction of mRNA molecules that share at least some of the sequence characteristics of dsRNA trigger molecules to which the cells have been exposed. RNAi has important biological roles in gene regulation and in protection of organisms against genetic parasites such as [[Virus|viruses]] and [[Transposon|transposons]]. | ||
The discovery of RNAi is a major technological breakthrough in biological research, perhaps as important as the development of the polymerase chain reaction ([[PCR]]), an in vitro technique that enables even tiny amounts of specific mRNAs to be measured easily. In experiments using RNAi in the fruit fly ''[[Drosophila melanogaster|Drosophila]]'' or in the roundworm ''[[Caenorhabditis elegans]]'', the effect of the loss of function of every known gene on a molecular pathway, cellular structure, or organism phenotype can now be determined rapidly and easily.<ref> Zamore PD (2006) Essay: RNA interference: big applause for silencing in Stockholm. Cell 127:1083-1086 doi:10.1016/j.cell.2006.12.001 PMID 17174883</ref> | |||
==Discovery of unexpected new regulatory roles for RNA== | |||
[[Image:C_elegans_RNAiA.jpg|left|frame|A ''C. elegans'' worm larva unable to shed its old cuticle after RNAi of the gene ''acn-1''. From: Ewer J, How the ecdysozoan changed its coat. PLoS Biol 3 e349 doi:10.1371/journal.pbio.0030349]] | |||
The revolutionary discovery of RNAi resulted from the unexpected outcome of experiments by plant scientists in the USA and the Netherlands.<ref>Napoli C ''et al.''(1990) [http://www.plantcell.org/cgi/reprint/2/4/279 Introduction of a chalcone synthase gene into Petunia results in reversible co-suppression of homologous genes ''in trans.''] Plant Cell 2: 279-89 PMID 12354959</ref> They were attempting to enhance the colors of [[petunia]] petals by introducing extra copies of a gene encoding an enzyme for flower pigmentation. While some plants with extra copies of the gene did show more intense colors, others surprisingly lost pigmentation (see figure). Analysis of the white petals showed that mRNA from both the endogenous gene and the newly introduced [[transgene]]s was absent (The term transgene refers to genes introduced into a plant by genetic manipulation from another species). Accordingly, the phenomenon was first called 'co-suppression of gene expression' but the molecular mechanism was unknown. | |||
<div class="thumb tright" style="background-color: #f9f9f9; border: 1px solid #CCCCCC; margin:0.5em;"> | |||
{|border="0" width=300px border="0" cellpadding="0" cellspacing="0" style="font-size: 85%; border: 1px solid #CCCCCC; margin: 0.3em;" | |||
|[[Image:PLoS_biology_234x60.GIF|300px]] | |||
|- | |||
|[[Image:Transcriptional_Silence.jpg|300px]] | |||
|} | |||
<div style="border: none; width:300px;"><div class="thumbcaption">'''Early Examples of Gene Silencing in Transgenic Plants.''' TGS: Normally when plants harboring separate transgenes encoding resistance to kanamycin (KAN) and hygromycin (HYG) are crossed, 50% of the progeny are resistant to the individual antibiotics and 25% are resistant to both (top). In cases of silencing, expression of the KAN marker is extinguished in the presence of the HYG marker, as indicated by only 25% KAN resistance and no double resistance (middle). PTGS: Transformation of wild-type petunia (bottom left) with a transgene encoding a pigment protein can lead to loss of pigment (white areas) owing to cosuppression of the transgene and homologous endogenous plant gene. (Photos left and middle provided by Jan Kooter and those on the right by Natalie Doetsch and Rich Jorgensen)<ref name="newparadigm">Matzke MA, Matzke AJM (2004) Planting the Seeds of a New Paradigm. PLoS Biol 2: e133 doi:[http://biology.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pbio.0020133 10.1371/journal.pbio.0020133]</ref></div></div></div> | |||
A few years later, plant virologists made a similar observation during experiments aimed at increasing plants' resistance to [[virus]]es. They knew that plants expressing virus-specific proteins could have enhanced tolerance, or even [[plant disease resistance|resistance]], to viral infection, but to their surprise they found the same effect using transgenes that only contained short regions of viral RNA sequences, too short to code for any viral protein. They concluded that viral RNA produced by transgenes could suppress virus activity and stop them from spreading throughout the plant. Reversing the experiment, by splicing short pieces of a plant gene into a plant virus, led to the discovery that these modified viruses could initiate the silencing of the specific plant gene. This 'virus-induced gene silencing' ('VIGS'), along with the cosupression phenomena have been collectively called [[post transcriptional gene silencing]] (PTGS), but currently the more usual scientific term for such phenomena is RNA interference.<ref name="newparadigm"/> | |||
Subsequently, many laboratories began to look for this phenomenon in other organisms and cosuppression was documented in ''C. elegans'' and Drosophila.<ref>Pal-Bhadra M ''et al.'' (1997) Cosuppression in ''Drosophila'': gene silencing of Alcohol dehydrogenase by | |||
white-Adh transgenes is Polycomb dependent. Cell 90:479-90 PMID 9267028 | |||
*Guo S, and Kemphues KJ. (1995) par-1, a gene required for establishing polarity in ''C. elegans'' embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 81:611-20 PMID 7758115 | |||
:*This paper describes the phenomenon that sense RNA was as effective as antisense RNA for silencing the gene expression in ''C. elegans''</ref> | |||
In 1998, Andrew Fire and Craig Mello coined the term ''RNAi'' when they made a particularly notable discovery that the injection of double-stranded RNA into ''C. elegans'' led to a potent and specific gene silencing effect.<ref>Fire A ''et al'' (1998) [http://www.nature.com/cgi-taf/DynaPage.taf?file=/nature/journal/v391/n6669/full/391806a0_r.html Potent and specific genetic interference by double-stranded RNA in ''Caenorhabditis elegans''] Nature 391:806-11 PMID 9486653</ref> This represented the first identification of the causative agent (double stranded RNA) of this hereto inexplicable phenomenon. In October 2006, Fire and Mello won the Nobel Prize for Medicine for their discoveries on gene silencing by RNAi.<ref>Baneholt B (2006) [http://nobelprize.org/nobel_prizes/medicine/laureates/2006/adv.html Advanced Information: RNA interference] Review for the 2006 Nobel Prize in Physiology or Medicine. Accessed 7 February 2007</ref> | |||
==Guide RNAs help proteins silence genes with a variety of flourishes == | |||
RNAi is but one of a group of mechanistically related gene [[RNA silencing|silencing]] phenomena which share a number of distinctive features. In these 'RNA silencing' mechanisms, small single strands of RNA, appropriately called 'guide' RNA, silence gene expression by guiding protein complexes to the final target sites where gene expression is altered. The steps leading to generating these 'guide' molecules, all involve an early initiation event triggered by double-stranded region in larger RNA molecules, and as discussed [[RNA_interference#Cellular_and_molecular_mechanisms|here]] involve the same cellular proteins (including [[ribonuclease]] enzymes named 'Dicer' and 'Argonaute'). | |||
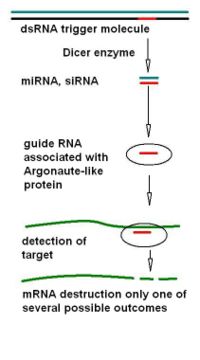
[[Image: | [[Image:RNAiSIMPLIFIED3.jpg|thumb|200px|left|Simplified scheme for common events in RNA interference; dsRNA, double stranded RNA; Dicer, a ribonuclease enzyme that cleaves dsRNA; siRNA, short interfering RNA; miRNA, micro RNA; mRNA, messenger RNA, for further details see text]] | ||
RNA silencing pathways exist in animals, plants, protists, and fungi, and there are many variations in the final outcome, but they share a common remarkable mechanistic feature of a partnership between a guide RNA molecule and an Argonaute-like protein that ultimately modifies gene activity. The final outcomes vary, for instance in which other cell components are involved and whether or DNA or mRNA is the main target for silencing. Despite their confusing variety (made more confusing by the different naming conventions used in different organisms), these pathways are all initiated by a larger double-stranded RNA trigger molecule, all involve the same types of specialized ribonuclease enzymes in generation of the small guide RNA, and all involve participation of an Argonaute-like protein guided by a small single strand of guide RNA to recognize a particular target, suggesting a common origin early in evolution. | |||
To better understand current scientific discussions of RNAi it is helpful to remember that that there are two mechanistically distinct class of RNA-mediated gene silencing events recognized today. The first type is where a block to gene activity prevents formation of mRNA. Gene silencing by methylation of DNA<ref>Wassenegger M ''et al.'' (1994) RNA-directed de novo methylation of genomic sequences in plants. Cell. 76:567-76. PMID 8313476</ref> or histones are processes in this class.<ref name="newparadigm"/> | |||
In the second class of RNA-mediated gene silencing mechanism, gene silencing results from interference with mRNA's cellular functions. Although in the past such events have been denoted as 'post-transcriptional gene silencing' (PTGS), they are now now simply called RNAi. (See [[RNA silencing]], [[RNA interference#Cellular and molecular mechanisms|Cellular and molecular mechanisms]], and Matzke and Matzke (2004)<ref name="newparadigm"/> for details.) | |||
The deliberate use of RNAi by plant scientists to reduce gene expression in plants (now usually called gene 'knockdown') has become common in recent years. Single-stranded [[antisense RNA]] that hybridized to a complementary, single-stranded, sense mRNA was deliberately introduced into plant cells to achieve knockdown. While plant biologists first believed that the resulting dsRNA helix could not be translated into a protein, it is now clear that the dsRNA triggered a RNAi response. The experimental use of dsRNA in biological research became more widespread after the discovery of the RNAi machinery, first in petunias and later in ''C. elegans''. | |||
== | ==Biological origins and roles== | ||
It has been suggested that the basic RNAi machinery was present is the common ancestor of all eukaryotic cells with an ancestral role in defence against genetic parasites.<ref>Cerutti H, Casas-Mollano JA (2006) On the origin and functions of RNA-mediated silencing: from protists to man.Curr Genet 50:81-99 PMID 16691418 </ref> | |||
In current day organisms the RNAi pathway does indeed play a role in defending against viruses and other foreign genetic material, both in animals (as part of the [[immune]] response) but especially in plants where it may protect against the self-propagation of parasitic or selfish DNA such as [[transposon]]s. The pathway is conserved across all [[eukaryote]]s, although it has been independently recruited to play other functions such as [[histone]] modification, the reorganization of genomic regions with complementary sequence to induce [[heterochromatin]] formation, and maintenance of [[centromere|centromeric]] heterochromatin.<ref>Saito K ''et al'' (2006) Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev 20:2214-22 PMID 16882972 | |||
* Stram Y, Kuzntzova L (2006) Inhibition of viruses by RNA interference. Virus Genes 32:299-306 PMID 16732482 | |||
* Tijsterman M ''et al.'' (2002) The genetics of RNA silencing. Ann Rev Genet 36:489-519 PMID 12429701 | |||
* Cerutti H, Casas-Mollano JA (2006) On the origin and functions of RNA-mediated silencing: from protists to man. Curr Genet 50:81-99 PMID 16691418 | |||
* Holmquist GP, Ashley T (2006) Chromosome organization and chromatin modification: influence on genome function and evolution. Cytogenet Genome Res 114:96-125 PMID 16825762 | |||
* Volpe TA ''et al'' (2002) Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297:1833-7 PMID 12193640</ref> | |||
===Primary-miRNAs are precursors=== | |||
The native cellular RNAi machinery is used by both plants and animals to regulate groups of tens or even hundreds of cellular genes via key regulatory genes that produce natural substrates for the Dicer enzyme. These natural Dicer substrates are called primary-[[micro RNA]]s. For producing [[micro RNA]]s (miRNAs), certain parts of the genome are [[Transcription (genetics)|transcribed]] into relatively short single-stranded RNA molecules that fold back on themselves in a hairpin shape to create a region with double stranded primary miRNA structure ([[miRNA|pri-miRNA]]). By 2006, thousands of miRNAs had been identified in plants and animals, including more than 470 in humans. | |||
===Cleavage of mRNA or interference with translation are both possible outcomes=== | |||
The Dicer enzyme then cuts 20-25 nucleotides from the base of the pri-miRNA hairpin to release the mature miRNA. If base-pairing with the target is ''perfect'' or near-perfect this may result in cleavage of messenger RNA ([[mRNA]]). This is quite similar to the events triggered in the cell by siRNA, however many miRNA's will base pair with mRNA with an ''imperfect'' match. In such cases, the miRNA causes the inhibition of translation and prevents normal function. Consequently, the RNAi machinery is important to regulate endogenous gene activity. This effect was first described for the ''C. elegans'' in 1993 by R. C. Lee ''et al'' of Harvard University. In plants, this mechanism was first shown in the "JAW microRNA" of ''[[Arabidopsis thaliana|Arabidopsis]]''; it is involved in regulating several genes that control the plant's shape. Genes have been found in bacteria that are similar in the sense that they control mRNA abundance or translation by binding an mRNA by base pairing, however they are not generally considered to be miRNA's because the Dicer enzyme is not involved.<ref>Lee RC ''et al'' (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843-54 PMID 8252621 | |||
* Palatnik JF ''et al'' (2003). Control of leaf morphogenesis by microRNAs. Nature 425:257-63 PMID 12931144 | |||
* Morita T ''et al'' (2006) Translational repression is sufficient for gene silencing by bacterial small noncoding RNAs in the absence of mRNA destruction. Proc Natl Acad Sci USA. 103:4858-63 PMID 16549791</ref> | |||
==Gene knockdown== | ===Gene knockdown=== | ||
[[Image:Drosophila_melanogaster_side_aka.jpg|thumb|300px|right|The fly ''Drosophila melanogaster'', image by André Karwath only for use at this webpage under [http://creativecommons.org/licenses/by-sa/2.5/ Creative Commons license]]] | |||
RNAi has recently been used to study the function of genes in [[model organism]]s. Double-stranded RNA for a gene of interest is introduced into a cell or organism, where, by RNAi, it often drastically reduces production of the protein that the gene codes for. Studying the effects of this can yield insights into the protein's role and function. As RNAi may not totally abolish expression of the gene, this is sometimes referred as a '[[Gene knockdown|knockdown]]', to distinguish it from '[[Gene knockout|knockout]]' in which expression of a gene is entirely eliminated by removing or destroying its DNA sequence. | |||
Most [[functional genomics]] applications of RNAi have used the [[nematode]] ''C. elegans'' and the fruit fly ''[[Drosophila melanogaster]]'', both of which are commonly used as 'model organisms' in genetics research.<ref>Dzitoyeva S ''et al'' (2003) Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence. Proc Natl Acad Sci USA 100:5485-90 PMID 12692303</ref> ''C. elegans'' is particularly useful for RNAi research because the effects of the gene silencing are generally heritable and because delivery of the dsRNA is exceptionally easy. Via a mechanism whose details are poorly understood, bacteria such as ''[[Escherichia coli]]'' that carry the desired dsRNA can be fed to the worms and will transfer the RNA to the worm via the intestinal tract. This 'delivery by feeding' yields essentially the same magnitude of gene silencing as do more costly and time-consuming traditional delivery methods, such as soaking the worms in dsRNA solution and injecting dsRNA into the gonads.<ref>Fortunato A, Fraser AG (2005) Uncover genetic interactions in ''Caenorhabditis elegans'' by RNA interference. Biosci Rep 25:299-307 PMID 16307378</ref> | |||
Another speculative use of dsRNA is in the repression of essential genes in eukaryotic human pathogens or viruses that are dissimilar from any human genes; this would be analogous to how existing drugs work. | ===Role in medicine=== | ||
The [[dsRNA]]s that trigger RNAi might be usable as drugs. The first application to reach [[clinical trial]]s is in the treatment of [[macular degeneration]]. RNAi has also proved able to completely reverse induced liver failure in mouse models (laboratory mice in which liver failure has been induced experimentally). Another speculative use of dsRNA is in the repression of essential genes in eukaryotic human pathogens or viruses that are dissimilar from any human genes; this would be analogous to how existing drugs work. | |||
RNAi interferes with the translation process of [[gene expression]] and appears not to interact with the DNA itself. Proponents of therapies based on RNAi suggest that the lack of interaction with | RNAi interferes with the translation process of [[gene expression]] and appears not to interact with the DNA itself. Proponents of therapies based on RNAi suggest that the lack of interaction with DNA might alleviate some patients' concerns about alteration of their DNA (as practiced in [[gene therapy]]), and suggest that this treatment would likely be no more feared than taking any prescription drug. For this reason RNAi and therapies based on RNAi have attracted much interest in the [[pharmaceutical]] and [[biotechnology]] industries. More recently, RNAi researchers have used RNAi to silence the expression of the human immunodeficiency virus ([[HIV]]) in mice. | ||
===Role in plant science=== | |||
RNAi is widely used to probe gene functions in plants. An example is a recent study from the laboratory of Jorge Dubcovsky where it was necessary to determine the function of a gene GPC-B1 that was thought to be involved in regulating wheat leaf senescence (and to affect cereal protein content).<ref>Pat Bailey (Jorge Dubcovsky) (2006). | |||
[http://www.eurekalert.org/pub_releases/2006-11/uoc--wgm111706.php Wheat gene may boost foods' nutrient content.] Accessed 7 February 2007. | |||
*Uauy C ''et al'' (2006). A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314:1298-1301 DOI: 10.1126/science.1133649 PMID 17124321</ref>. This laboratory used RNAi to knockdown expression of the GPC-B1 gene in cereal wheat and found spectacular changes in grain germination after knockdown. The GPC-B1 knockdown wheat variety showed 30% less grain protein, zinc and iron, without differences in grain size, and verified that a single gene was responsible for all the effects. They suggest that increased expression of the gene cam improve grain protein content and nutritional value. | |||
More practically, Australia's CSIRO has developed a new experimental wheat variety with the potential to provide benefits in the areas of bowel health, diabetes and obesity. In this case, RNAi was used in wheat to increase the content of amylose, a form of starch that is more resistant to digestion.<ref>CSIRO (2006) [http://www.csiro.au/files/files/pahk.pdf Partnerships give new wheats a healthy future. Gene silencing technology and conventional plant breeding are being used to develop wheat with significant human health benefits.] Accessed 7 February 2007.</ref> | |||
CSIRO and others have argued that cisgenic plants - that is plants created by genetic manipulation using RNAi (which they dub 'GM-lite')- pose less risks than addition of genes from other species (transgenics) as (they argue) new proteins are unlikely to be produced.<ref>Schouten HJ ''et al.'' (2006) Do cisgenic plants warrant less stringent oversight? Nat Biotechnol. 2006 24:753 PMID 16841052. This letter argues there are strong reasons for legislators to differentiate cisgenic (GM lite) from transgenic plants. Counterarguments are indexed in the PMID link.</ref> | |||
==Cellular and molecular mechanisms== | |||
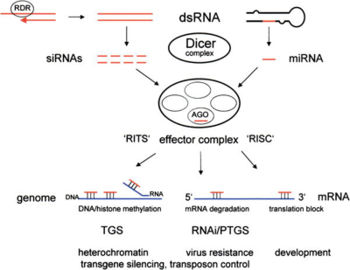
[[Image:RNAsilenceOVERVIEW.jpg|thumb|left|350px|Short RNAs derived from Dicer cleavage of dsRNA are incorporated into multi-protein effector complexes, referred to in the text as RISC, but also including RITS (RNA-induced initiation of TGS) to target mRNA degradation (RNAi/PTGS), translation inhibition, or TGS and genome modifications. Argonaute (AGO) proteins bind short RNAs and ‘shepherd’ them as ''guide'' RNAs to appropriate effector complexes. Target nucleic acids are in blue, short RNAs in red, proteins and enzyme complexes as ovals.<ref name="newparadigm"/>]] | |||
<div class="thumb tright" style="background-color: #f9f9f9; border: 1px solid #CCCCCC; margin:0.5em;"> | |||
{|border="0" width=200px border="0" cellpadding="0" cellspacing="0" style="border: 1px solid #CCCCCC; margin: 0.3em;" | |||
|[[Image:Dicer_Giardia.jpg|200px]] | |||
|} | |||
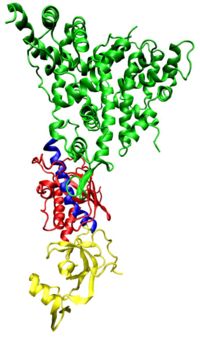
<div style="border: none; width:200px;"><div class="thumbcaption">One molecule of the Dicer protein from the single celled protist ''Giardia intestinalis'', which catalyzes the cleavage of dsRNA to siRNAs. The ribonuclease (RNAase) enzyme domains are colored green, the PAZ domain yellow, the platform domain red, and the connector helix blue. The distance between the RNase and PAZ domains, determined by the length and angle of the connector helix, has been suggested as the determinant for the length of siRNA molecules produced by a given Dicer variant.(After Wikipedia figure caption in the RNAi article).</div></div> | |||
{|border="0" width=200px border="0" cellpadding="0" cellspacing="0" style="border: 1px solid #CCCCCC; margin: 0.3em;" | |||
|[[Image:RNAipolymerase.jpg|200px]] | |||
|} | |||
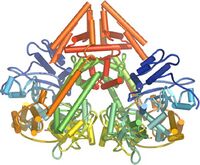
<div style="border: none; width:200px;"><div class="thumbcaption">Schematic showing the fold of the QDE-1 RNA interference polymerase. The dimeric molecule is shown with the polypeptide chains colored from blue at the N termini to red at the C termini. <ref name="jones">Jones R (2006) RNA silencing sheds light on the RNA world. PLoS Biol 4(12): e448 doi:[http://biology.plosjournals.org/perlserv/?request=get-document&doi=10.1371/journal.pbio.0040448 10.1371/journal.pbio.0040448]</ref></div></div></div> | |||
===Double-stranded regions of RNA are the triggers for RNAi=== | |||
There are two types of triggers for RNAi. These (1) fully double stranded RNA molecules ([[dsRNA]]), and (2) 'hairpin' forms of single-stranded RNA molecules in which the 'stem' of the RNA hairpin structure has complementary RNA base-pairing between two different parts of the same RNA strand (see figure to left). These two types of trigger structure can both be substrates for a [[ribonuclease]] enzyme that is appropriately named '[[Dicer]]'. | |||
===Dicer=== | |||
The RNAi process requires active participation of cellular machinery, and the properties of the Dicer enzyme are important for understanding this process. | |||
Dicer has two active sites, both able to cleave its substrate RNAs. These active sites are separated some distance from one another in the Dicer enzyme (see figure to the right) and this explains the size of the RNA fragments they produce when they act on a double stranded RNA ([[dsRNA]]) target, a ''dicing'' process which is an early step in the cellular pathway that brings about RNAi.<ref>Bernstein E ''et al.'' (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363-6 PMID 11201747. Comment in Baulcombe D (2001) RNA silencing. Diced defence. Nature 409:295-6 | |||
* Hammond SM ''et al'' (2001) Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293:1146–50 PMID 11498593 | |||
* Nicholson RH and Nicholson AW (2002) Molecular characterization of a mouse cDNA encoding Dicer, a ribonuclease III ortholog involved in RNA interference. Mamm Genome 13:67-73. PMID 11889553 | |||
* Hammond SM ''et al.'' (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293-6. PMID 10749213 | |||
* Tabara H, ''et al.'' (2002) The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in ''C. elegans''. Cell 109:861-71. PMID 12110183 | |||
* Liu Q ''et al.'' (2003) R2D2, a bridge between the initiation and effector steps of the ''Drosophila''. RNAi pathway Science 301:1921-5. PMID 14512631 | |||
* Macrae IJ ''et al.'' (2006) Structural basis for double-stranded RNA processing by Dicer. Science 311:195-8. PMID 16410517</ref> | |||
===Multi-protein transcriptional silencing complexes generate the ''guide'' strand RNA=== | |||
Dicer binds to and cleaves short double-stranded trigger RNA molecules ([[dsRNA]]) in two positions to produce double-stranded fragments of 21-23 [[base pair]]s with two-base single-stranded overhangs on each end. The short double-stranded fragments produced by Dicer when it acts on dsRNA are called small interfering RNAs ([[siRNA]]s), or [[Micro RNA|miRNA]] when it acts of the cell's own transcribed, hairpin-forming miRNA precursors. One RNA strand - the ''guide'' strand - from these siRNAs.<ref>Tomari Y ''et al'' (2004) [http://www.sciencemag.org/cgi/content/abstract/306/5700/1377?ijkey=9868c8149b22bb60769a904684c1ac397aeecb73&keytype2=tf_ipsecsha A protein sensor for siRNA asymmetry.] Science 306:1377-80. | |||
*Schwarz DS ''et al'' (2003) Asymmetry in the assembly of the RNAi enzyme complex. Cell 115:199-208 PMID 14567917</ref> is generated by an aggregate of several proteins, including DICER, called the RNA-induced silencing complex ([[RNA-induced_silencing_complex|RISC]]).<ref name="lodish">Lodish H ''et al.'' (2004) ''Molecular Cell Biology'' 5th ed. ISBN 0716743663. Search this text and others online [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Books here].</ref> | |||
===Cellular RNA-dependent RNA polymerase can also produce double-stranded triggers=== | |||
In plants, protozoa, fungi, and nematodes, a cell-encoded RNA-dependent RNA polymerase (cRdRp, RDR; see figures) produces fully dsRNA trigger molecules for RNA from single-stranded RNA. The structure of cell-encoded RNA-dependent RNA polymerase from ''Neurospora crassa'' (shown to right) is a relatively compact dimeric molecule and its core is a catalytic apparatus and protein folding that is strikingly similar to the catalytic core of the DNA-dependent RNA polymerases responsible for transcription.<ref name="jones"/> | |||
===Argonautes are slicers that cleave mRNA with a little help from their friends=== | |||
The RISC complex containing Dicer and Argonaute has an important role in processing trigger molecules to generate the short single stranded effectors of interference, termed the 'guide strand', and in degrading the other stand, termed the 'passenger strand'. The human RISC complex shows a nearly 10-fold greater activity using the pre-miRNA Dicer substrate over duplex siRNA. RISC can distinguish the guide strand of the siRNA from the passenger strand, and specifically incorporates only the guide strand.<ref>Gregory RI (2005) RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 123:631-40 PMID 16271387</ref> | |||
After integration into the RISC, single guide strands of RNA, either from siRNAs or miRNAs, bound to Argonaute-like proteins, can move to the target sites for gene silencing. One outcome that can occur at the target site is that the guide RNA can base pair to target mRNA and induce the RISC component protein Argonaute to cleave it, thereby preventing it from being used as a translation template. Proteins with a similar sequence to Argonaute (e.g. RDE-1, P-element associated wimpy testes (Piwi)) are present in nearly every eukaryote, from fungi to plants, flies, and mammals, often as gene families <ref>Girard A ''et al.'' (2006) A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442:199-202 PMID 16751776 | |||
*Baumberger N, Baulcombe DC (2005) ''Arabidopsis'' ARGONAUTE1 is an RNA slicer that selectively recruits microRNAs and short interfering RNAs. Proc Natl Acad Sci USA 102:11928-33. PMID 16081530</ref>. | |||
Organisms vary in their cells' ability to take up foreign dsRNA and use it in the RNAi pathway. The effects of RNAi are both systemic and heritable in plants and in ''C. elegans'', although not in ''Drosophila'' or mammals because of the absence of [[RNA replicase]] in these organisms. In plants, RNAi is thought to propagate through cells via the transfer of siRNAs through [[plasmodesmata]].<ref name="lodish" /> | |||
==References== | ==References== | ||
===Citations=== | ===Citations=== | ||
<div class="references-small" style="-moz-column-count:2; -webkit-column-count:2; column-count:2;"> | |||
<references /> | <references /> | ||
</div> | |||
===Further reading=== | ===Further reading=== | ||
*Ahlquist | *Ahlquist P (2002) RNA-dependent RNA polymerases, viruses, and RNA silencing. Science 296:1270-73 | ||
*Baulcombe D (2004) RNA silencing in plants. Nature | *Baulcombe D (2004) RNA silencing in plants. Nature 31:356–63 | ||
*Cogoni C, Macino G (1999) Gene silencing in ''Neurospora crassa'' requires a protein homologous to RNA-dependent RNA polymerase. Nature 399: | *Cogoni C, Macino G (1999) Gene silencing in ''Neurospora crassa'' requires a protein homologous to RNA-dependent RNA polymerase. Nature 399:166–9 | ||
* | *Lippman Z, Martienssen R (2004) The role of RNA interference in heterochromatic silencing. Nature 431:364–70. | ||
*Makeyev EV, Bamford DH (2002) Cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing has two distinct activity modes. Mol Cell 10: | *Makeyev EV, Bamford DH (2002) Cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing has two distinct activity modes. Mol Cell 10:1417–27 | ||
*Matzke | *Matzke M ''et al.'' (2001) RNA: guiding gene silencing. Science 293:1080-3 | ||
*Mello CC, Conte D Jr (2004) Revealing the world of RNA interference. Nature 431 | *Mello CC, Conte D Jr (2004) Revealing the world of RNA interference. Nature 431:338–42 | ||
*Sharp | *Sharp PA (2001) RNA interference--2001. Genes Dev 15:485-90 | ||
*Sijen T | *Sijen T ''et al'' (2001) On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107:465–76 | ||
===See also=== | ===See also=== | ||
*[[The central dogma]] | |||
*[[RNA silencing]] | |||
*[[Small interfering RNA]] | *[[Small interfering RNA]] | ||
*[[micro RNA]] | *[[micro RNA]] | ||
*[[Biotechnology and | *[[Biotechnology and plant breeding]] | ||
===External links=== | ===External links=== | ||
*[http://www.nature.com/focus/rnai/animations/animation/animation.htm Animation of the RNAi process], from science magazine ''[[Nature (journal)|Nature]]''. | |||
*[http://www.nature.com/focus/rnai/animations/animation/animation.htm Animation of the RNAi process], from ''[[Nature (journal)|Nature]]'' | |||
*[http://www.rnainterference.org/ siRNA Database] | *[http://www.rnainterference.org/ siRNA Database] | ||
*[http://www.pbs.org/wgbh/nova/sciencenow/3210/02.html NOVA scienceNOW explains RNAi] - A 15 minute video of the ''[[Nova (TV series)|Nova]]'' broadcast that aired on [[PBS]], | *[http://www.pbs.org/wgbh/nova/sciencenow/3210/02.html NOVA scienceNOW explains RNAi] - A 15 minute video of the ''[[Nova (TV series)|Nova]]'' broadcast that aired on [[PBS]], July 26, 2005. | ||
*[http://nematoda.bio.nyu.edu/cgi-bin/rnai/index.cgi RNA interference (RNAi) Database] | *[http://nematoda.bio.nyu.edu/cgi-bin/rnai/index.cgi RNA interference (RNAi) Database] | ||
*[http:// | * The [http://en.wikipedia.org/wiki/RNA_interference Wikipedia article] (accessed 12 February) on this topic is of excellent quality and is possibly best read by neophytes in RNAi matters after reading this Citizendium version. | ||
<br/> | <br/> | ||
Latest revision as of 17:45, 2 October 2013
RNA interference (RNAi) is a process that inhibits the flow of genetic information to protein synthesis, which normally proceeds from deoxyribonucleic acid (DNA) to messenger ribonucleic acid (mRNA) to proteins - a process known as gene expression (see figure, top right). mRNA, a single-stranded polymer, is the key molecule whose sequence of nucleotides transports the information encoded in DNA to regions of the cell where that DNA-genetic information is used for synthesizing proteins. Understanding that flow of information is necessary for understanding the RNAi mediated feedback loops that inhibit gene expression.
Specifically, RNAi is a mechanism in eukaryotic cells that is triggered when such cells are exposed to certain double-stranded ribonucleic acid (dsRNA) molecules. The process has been detected in many organisms, including animal, plant and protist cells. The distinguishing characteristic of RNAi is destruction of mRNA molecules that share at least some of the sequence characteristics of dsRNA trigger molecules to which the cells have been exposed. RNAi has important biological roles in gene regulation and in protection of organisms against genetic parasites such as viruses and transposons.
The discovery of RNAi is a major technological breakthrough in biological research, perhaps as important as the development of the polymerase chain reaction (PCR), an in vitro technique that enables even tiny amounts of specific mRNAs to be measured easily. In experiments using RNAi in the fruit fly Drosophila or in the roundworm Caenorhabditis elegans, the effect of the loss of function of every known gene on a molecular pathway, cellular structure, or organism phenotype can now be determined rapidly and easily.[1]
Discovery of unexpected new regulatory roles for RNA
The revolutionary discovery of RNAi resulted from the unexpected outcome of experiments by plant scientists in the USA and the Netherlands.[2] They were attempting to enhance the colors of petunia petals by introducing extra copies of a gene encoding an enzyme for flower pigmentation. While some plants with extra copies of the gene did show more intense colors, others surprisingly lost pigmentation (see figure). Analysis of the white petals showed that mRNA from both the endogenous gene and the newly introduced transgenes was absent (The term transgene refers to genes introduced into a plant by genetic manipulation from another species). Accordingly, the phenomenon was first called 'co-suppression of gene expression' but the molecular mechanism was unknown.

|

|
A few years later, plant virologists made a similar observation during experiments aimed at increasing plants' resistance to viruses. They knew that plants expressing virus-specific proteins could have enhanced tolerance, or even resistance, to viral infection, but to their surprise they found the same effect using transgenes that only contained short regions of viral RNA sequences, too short to code for any viral protein. They concluded that viral RNA produced by transgenes could suppress virus activity and stop them from spreading throughout the plant. Reversing the experiment, by splicing short pieces of a plant gene into a plant virus, led to the discovery that these modified viruses could initiate the silencing of the specific plant gene. This 'virus-induced gene silencing' ('VIGS'), along with the cosupression phenomena have been collectively called post transcriptional gene silencing (PTGS), but currently the more usual scientific term for such phenomena is RNA interference.[3]
Subsequently, many laboratories began to look for this phenomenon in other organisms and cosuppression was documented in C. elegans and Drosophila.[4] In 1998, Andrew Fire and Craig Mello coined the term RNAi when they made a particularly notable discovery that the injection of double-stranded RNA into C. elegans led to a potent and specific gene silencing effect.[5] This represented the first identification of the causative agent (double stranded RNA) of this hereto inexplicable phenomenon. In October 2006, Fire and Mello won the Nobel Prize for Medicine for their discoveries on gene silencing by RNAi.[6]
Guide RNAs help proteins silence genes with a variety of flourishes
RNAi is but one of a group of mechanistically related gene silencing phenomena which share a number of distinctive features. In these 'RNA silencing' mechanisms, small single strands of RNA, appropriately called 'guide' RNA, silence gene expression by guiding protein complexes to the final target sites where gene expression is altered. The steps leading to generating these 'guide' molecules, all involve an early initiation event triggered by double-stranded region in larger RNA molecules, and as discussed here involve the same cellular proteins (including ribonuclease enzymes named 'Dicer' and 'Argonaute').
RNA silencing pathways exist in animals, plants, protists, and fungi, and there are many variations in the final outcome, but they share a common remarkable mechanistic feature of a partnership between a guide RNA molecule and an Argonaute-like protein that ultimately modifies gene activity. The final outcomes vary, for instance in which other cell components are involved and whether or DNA or mRNA is the main target for silencing. Despite their confusing variety (made more confusing by the different naming conventions used in different organisms), these pathways are all initiated by a larger double-stranded RNA trigger molecule, all involve the same types of specialized ribonuclease enzymes in generation of the small guide RNA, and all involve participation of an Argonaute-like protein guided by a small single strand of guide RNA to recognize a particular target, suggesting a common origin early in evolution.
To better understand current scientific discussions of RNAi it is helpful to remember that that there are two mechanistically distinct class of RNA-mediated gene silencing events recognized today. The first type is where a block to gene activity prevents formation of mRNA. Gene silencing by methylation of DNA[7] or histones are processes in this class.[3]
In the second class of RNA-mediated gene silencing mechanism, gene silencing results from interference with mRNA's cellular functions. Although in the past such events have been denoted as 'post-transcriptional gene silencing' (PTGS), they are now now simply called RNAi. (See RNA silencing, Cellular and molecular mechanisms, and Matzke and Matzke (2004)[3] for details.)
The deliberate use of RNAi by plant scientists to reduce gene expression in plants (now usually called gene 'knockdown') has become common in recent years. Single-stranded antisense RNA that hybridized to a complementary, single-stranded, sense mRNA was deliberately introduced into plant cells to achieve knockdown. While plant biologists first believed that the resulting dsRNA helix could not be translated into a protein, it is now clear that the dsRNA triggered a RNAi response. The experimental use of dsRNA in biological research became more widespread after the discovery of the RNAi machinery, first in petunias and later in C. elegans.
Biological origins and roles
It has been suggested that the basic RNAi machinery was present is the common ancestor of all eukaryotic cells with an ancestral role in defence against genetic parasites.[8] In current day organisms the RNAi pathway does indeed play a role in defending against viruses and other foreign genetic material, both in animals (as part of the immune response) but especially in plants where it may protect against the self-propagation of parasitic or selfish DNA such as transposons. The pathway is conserved across all eukaryotes, although it has been independently recruited to play other functions such as histone modification, the reorganization of genomic regions with complementary sequence to induce heterochromatin formation, and maintenance of centromeric heterochromatin.[9]
Primary-miRNAs are precursors
The native cellular RNAi machinery is used by both plants and animals to regulate groups of tens or even hundreds of cellular genes via key regulatory genes that produce natural substrates for the Dicer enzyme. These natural Dicer substrates are called primary-micro RNAs. For producing micro RNAs (miRNAs), certain parts of the genome are transcribed into relatively short single-stranded RNA molecules that fold back on themselves in a hairpin shape to create a region with double stranded primary miRNA structure (pri-miRNA). By 2006, thousands of miRNAs had been identified in plants and animals, including more than 470 in humans.
Cleavage of mRNA or interference with translation are both possible outcomes
The Dicer enzyme then cuts 20-25 nucleotides from the base of the pri-miRNA hairpin to release the mature miRNA. If base-pairing with the target is perfect or near-perfect this may result in cleavage of messenger RNA (mRNA). This is quite similar to the events triggered in the cell by siRNA, however many miRNA's will base pair with mRNA with an imperfect match. In such cases, the miRNA causes the inhibition of translation and prevents normal function. Consequently, the RNAi machinery is important to regulate endogenous gene activity. This effect was first described for the C. elegans in 1993 by R. C. Lee et al of Harvard University. In plants, this mechanism was first shown in the "JAW microRNA" of Arabidopsis; it is involved in regulating several genes that control the plant's shape. Genes have been found in bacteria that are similar in the sense that they control mRNA abundance or translation by binding an mRNA by base pairing, however they are not generally considered to be miRNA's because the Dicer enzyme is not involved.[10]
Gene knockdown

RNAi has recently been used to study the function of genes in model organisms. Double-stranded RNA for a gene of interest is introduced into a cell or organism, where, by RNAi, it often drastically reduces production of the protein that the gene codes for. Studying the effects of this can yield insights into the protein's role and function. As RNAi may not totally abolish expression of the gene, this is sometimes referred as a 'knockdown', to distinguish it from 'knockout' in which expression of a gene is entirely eliminated by removing or destroying its DNA sequence.
Most functional genomics applications of RNAi have used the nematode C. elegans and the fruit fly Drosophila melanogaster, both of which are commonly used as 'model organisms' in genetics research.[11] C. elegans is particularly useful for RNAi research because the effects of the gene silencing are generally heritable and because delivery of the dsRNA is exceptionally easy. Via a mechanism whose details are poorly understood, bacteria such as Escherichia coli that carry the desired dsRNA can be fed to the worms and will transfer the RNA to the worm via the intestinal tract. This 'delivery by feeding' yields essentially the same magnitude of gene silencing as do more costly and time-consuming traditional delivery methods, such as soaking the worms in dsRNA solution and injecting dsRNA into the gonads.[12]
Role in medicine
The dsRNAs that trigger RNAi might be usable as drugs. The first application to reach clinical trials is in the treatment of macular degeneration. RNAi has also proved able to completely reverse induced liver failure in mouse models (laboratory mice in which liver failure has been induced experimentally). Another speculative use of dsRNA is in the repression of essential genes in eukaryotic human pathogens or viruses that are dissimilar from any human genes; this would be analogous to how existing drugs work.
RNAi interferes with the translation process of gene expression and appears not to interact with the DNA itself. Proponents of therapies based on RNAi suggest that the lack of interaction with DNA might alleviate some patients' concerns about alteration of their DNA (as practiced in gene therapy), and suggest that this treatment would likely be no more feared than taking any prescription drug. For this reason RNAi and therapies based on RNAi have attracted much interest in the pharmaceutical and biotechnology industries. More recently, RNAi researchers have used RNAi to silence the expression of the human immunodeficiency virus (HIV) in mice.
Role in plant science
RNAi is widely used to probe gene functions in plants. An example is a recent study from the laboratory of Jorge Dubcovsky where it was necessary to determine the function of a gene GPC-B1 that was thought to be involved in regulating wheat leaf senescence (and to affect cereal protein content).[13]. This laboratory used RNAi to knockdown expression of the GPC-B1 gene in cereal wheat and found spectacular changes in grain germination after knockdown. The GPC-B1 knockdown wheat variety showed 30% less grain protein, zinc and iron, without differences in grain size, and verified that a single gene was responsible for all the effects. They suggest that increased expression of the gene cam improve grain protein content and nutritional value.
More practically, Australia's CSIRO has developed a new experimental wheat variety with the potential to provide benefits in the areas of bowel health, diabetes and obesity. In this case, RNAi was used in wheat to increase the content of amylose, a form of starch that is more resistant to digestion.[14]
CSIRO and others have argued that cisgenic plants - that is plants created by genetic manipulation using RNAi (which they dub 'GM-lite')- pose less risks than addition of genes from other species (transgenics) as (they argue) new proteins are unlikely to be produced.[15]
Cellular and molecular mechanisms
|
|
Double-stranded regions of RNA are the triggers for RNAi
There are two types of triggers for RNAi. These (1) fully double stranded RNA molecules (dsRNA), and (2) 'hairpin' forms of single-stranded RNA molecules in which the 'stem' of the RNA hairpin structure has complementary RNA base-pairing between two different parts of the same RNA strand (see figure to left). These two types of trigger structure can both be substrates for a ribonuclease enzyme that is appropriately named 'Dicer'.
Dicer
The RNAi process requires active participation of cellular machinery, and the properties of the Dicer enzyme are important for understanding this process.
Dicer has two active sites, both able to cleave its substrate RNAs. These active sites are separated some distance from one another in the Dicer enzyme (see figure to the right) and this explains the size of the RNA fragments they produce when they act on a double stranded RNA (dsRNA) target, a dicing process which is an early step in the cellular pathway that brings about RNAi.[17]
Multi-protein transcriptional silencing complexes generate the guide strand RNA
Dicer binds to and cleaves short double-stranded trigger RNA molecules (dsRNA) in two positions to produce double-stranded fragments of 21-23 base pairs with two-base single-stranded overhangs on each end. The short double-stranded fragments produced by Dicer when it acts on dsRNA are called small interfering RNAs (siRNAs), or miRNA when it acts of the cell's own transcribed, hairpin-forming miRNA precursors. One RNA strand - the guide strand - from these siRNAs.[18] is generated by an aggregate of several proteins, including DICER, called the RNA-induced silencing complex (RISC).[19]
Cellular RNA-dependent RNA polymerase can also produce double-stranded triggers
In plants, protozoa, fungi, and nematodes, a cell-encoded RNA-dependent RNA polymerase (cRdRp, RDR; see figures) produces fully dsRNA trigger molecules for RNA from single-stranded RNA. The structure of cell-encoded RNA-dependent RNA polymerase from Neurospora crassa (shown to right) is a relatively compact dimeric molecule and its core is a catalytic apparatus and protein folding that is strikingly similar to the catalytic core of the DNA-dependent RNA polymerases responsible for transcription.[16]
Argonautes are slicers that cleave mRNA with a little help from their friends
The RISC complex containing Dicer and Argonaute has an important role in processing trigger molecules to generate the short single stranded effectors of interference, termed the 'guide strand', and in degrading the other stand, termed the 'passenger strand'. The human RISC complex shows a nearly 10-fold greater activity using the pre-miRNA Dicer substrate over duplex siRNA. RISC can distinguish the guide strand of the siRNA from the passenger strand, and specifically incorporates only the guide strand.[20]
After integration into the RISC, single guide strands of RNA, either from siRNAs or miRNAs, bound to Argonaute-like proteins, can move to the target sites for gene silencing. One outcome that can occur at the target site is that the guide RNA can base pair to target mRNA and induce the RISC component protein Argonaute to cleave it, thereby preventing it from being used as a translation template. Proteins with a similar sequence to Argonaute (e.g. RDE-1, P-element associated wimpy testes (Piwi)) are present in nearly every eukaryote, from fungi to plants, flies, and mammals, often as gene families [21].
Organisms vary in their cells' ability to take up foreign dsRNA and use it in the RNAi pathway. The effects of RNAi are both systemic and heritable in plants and in C. elegans, although not in Drosophila or mammals because of the absence of RNA replicase in these organisms. In plants, RNAi is thought to propagate through cells via the transfer of siRNAs through plasmodesmata.[19]
References
Citations
- ↑ Zamore PD (2006) Essay: RNA interference: big applause for silencing in Stockholm. Cell 127:1083-1086 doi:10.1016/j.cell.2006.12.001 PMID 17174883
- ↑ Napoli C et al.(1990) Introduction of a chalcone synthase gene into Petunia results in reversible co-suppression of homologous genes in trans. Plant Cell 2: 279-89 PMID 12354959
- ↑ 3.0 3.1 3.2 3.3 3.4 Matzke MA, Matzke AJM (2004) Planting the Seeds of a New Paradigm. PLoS Biol 2: e133 doi:10.1371/journal.pbio.0020133
- ↑ Pal-Bhadra M et al. (1997) Cosuppression in Drosophila: gene silencing of Alcohol dehydrogenase by
white-Adh transgenes is Polycomb dependent. Cell 90:479-90 PMID 9267028
- Guo S, and Kemphues KJ. (1995) par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 81:611-20 PMID 7758115
- This paper describes the phenomenon that sense RNA was as effective as antisense RNA for silencing the gene expression in C. elegans
- ↑ Fire A et al (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans Nature 391:806-11 PMID 9486653
- ↑ Baneholt B (2006) Advanced Information: RNA interference Review for the 2006 Nobel Prize in Physiology or Medicine. Accessed 7 February 2007
- ↑ Wassenegger M et al. (1994) RNA-directed de novo methylation of genomic sequences in plants. Cell. 76:567-76. PMID 8313476
- ↑ Cerutti H, Casas-Mollano JA (2006) On the origin and functions of RNA-mediated silencing: from protists to man.Curr Genet 50:81-99 PMID 16691418
- ↑ Saito K et al (2006) Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev 20:2214-22 PMID 16882972
- Stram Y, Kuzntzova L (2006) Inhibition of viruses by RNA interference. Virus Genes 32:299-306 PMID 16732482
- Tijsterman M et al. (2002) The genetics of RNA silencing. Ann Rev Genet 36:489-519 PMID 12429701
- Cerutti H, Casas-Mollano JA (2006) On the origin and functions of RNA-mediated silencing: from protists to man. Curr Genet 50:81-99 PMID 16691418
- Holmquist GP, Ashley T (2006) Chromosome organization and chromatin modification: influence on genome function and evolution. Cytogenet Genome Res 114:96-125 PMID 16825762
- Volpe TA et al (2002) Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 297:1833-7 PMID 12193640
- ↑ Lee RC et al (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843-54 PMID 8252621
- Palatnik JF et al (2003). Control of leaf morphogenesis by microRNAs. Nature 425:257-63 PMID 12931144
- Morita T et al (2006) Translational repression is sufficient for gene silencing by bacterial small noncoding RNAs in the absence of mRNA destruction. Proc Natl Acad Sci USA. 103:4858-63 PMID 16549791
- ↑ Dzitoyeva S et al (2003) Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence. Proc Natl Acad Sci USA 100:5485-90 PMID 12692303
- ↑ Fortunato A, Fraser AG (2005) Uncover genetic interactions in Caenorhabditis elegans by RNA interference. Biosci Rep 25:299-307 PMID 16307378
- ↑ Pat Bailey (Jorge Dubcovsky) (2006).
Wheat gene may boost foods' nutrient content. Accessed 7 February 2007.
- Uauy C et al (2006). A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314:1298-1301 DOI: 10.1126/science.1133649 PMID 17124321
- ↑ CSIRO (2006) Partnerships give new wheats a healthy future. Gene silencing technology and conventional plant breeding are being used to develop wheat with significant human health benefits. Accessed 7 February 2007.
- ↑ Schouten HJ et al. (2006) Do cisgenic plants warrant less stringent oversight? Nat Biotechnol. 2006 24:753 PMID 16841052. This letter argues there are strong reasons for legislators to differentiate cisgenic (GM lite) from transgenic plants. Counterarguments are indexed in the PMID link.
- ↑ 16.0 16.1 Jones R (2006) RNA silencing sheds light on the RNA world. PLoS Biol 4(12): e448 doi:10.1371/journal.pbio.0040448
- ↑ Bernstein E et al. (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409:363-6 PMID 11201747. Comment in Baulcombe D (2001) RNA silencing. Diced defence. Nature 409:295-6
- Hammond SM et al (2001) Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293:1146–50 PMID 11498593
- Nicholson RH and Nicholson AW (2002) Molecular characterization of a mouse cDNA encoding Dicer, a ribonuclease III ortholog involved in RNA interference. Mamm Genome 13:67-73. PMID 11889553
- Hammond SM et al. (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404:293-6. PMID 10749213
- Tabara H, et al. (2002) The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell 109:861-71. PMID 12110183
- Liu Q et al. (2003) R2D2, a bridge between the initiation and effector steps of the Drosophila. RNAi pathway Science 301:1921-5. PMID 14512631
- Macrae IJ et al. (2006) Structural basis for double-stranded RNA processing by Dicer. Science 311:195-8. PMID 16410517
- ↑ Tomari Y et al (2004) A protein sensor for siRNA asymmetry. Science 306:1377-80.
- Schwarz DS et al (2003) Asymmetry in the assembly of the RNAi enzyme complex. Cell 115:199-208 PMID 14567917
- ↑ 19.0 19.1 Lodish H et al. (2004) Molecular Cell Biology 5th ed. ISBN 0716743663. Search this text and others online here.
- ↑ Gregory RI (2005) RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 123:631-40 PMID 16271387
- ↑ Girard A et al. (2006) A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442:199-202 PMID 16751776
- Baumberger N, Baulcombe DC (2005) Arabidopsis ARGONAUTE1 is an RNA slicer that selectively recruits microRNAs and short interfering RNAs. Proc Natl Acad Sci USA 102:11928-33. PMID 16081530
Further reading
- Ahlquist P (2002) RNA-dependent RNA polymerases, viruses, and RNA silencing. Science 296:1270-73
- Baulcombe D (2004) RNA silencing in plants. Nature 31:356–63
- Cogoni C, Macino G (1999) Gene silencing in Neurospora crassa requires a protein homologous to RNA-dependent RNA polymerase. Nature 399:166–9
- Lippman Z, Martienssen R (2004) The role of RNA interference in heterochromatic silencing. Nature 431:364–70.
- Makeyev EV, Bamford DH (2002) Cellular RNA-dependent RNA polymerase involved in posttranscriptional gene silencing has two distinct activity modes. Mol Cell 10:1417–27
- Matzke M et al. (2001) RNA: guiding gene silencing. Science 293:1080-3
- Mello CC, Conte D Jr (2004) Revealing the world of RNA interference. Nature 431:338–42
- Sharp PA (2001) RNA interference--2001. Genes Dev 15:485-90
- Sijen T et al (2001) On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107:465–76
See also
External links
- Animation of the RNAi process, from science magazine Nature.
- siRNA Database
- NOVA scienceNOW explains RNAi - A 15 minute video of the Nova broadcast that aired on PBS, July 26, 2005.
- RNA interference (RNAi) Database
- The Wikipedia article (accessed 12 February) on this topic is of excellent quality and is possibly best read by neophytes in RNAi matters after reading this Citizendium version.
- Pages using PMID magic links
- Pages using ISBN magic links
- Subpages
- Biology Extra Subpages
- Health Sciences Extra Subpages
- Biology Approved Extra Subpages
- Health Sciences Approved Extra Subpages
- Citable versions of articles
- Biology Citable Version Subpages
- Health Sciences Citable Version Subpages
- All Content
- Biology Content
- Health Sciences Content