Flucloxacillin: Difference between revisions
imported>David E. Volk m (→External links) |
imported>David E. Volk (chem infobox, names and brands column form) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
[[Image:Flucloxacillin structure.jpg| | |||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Flucloxacillin structure.jpg|center|thumb|250px|{{#ifexist:Template:Flucloxacillin structure.jpg/credit|{{Flucloxacillin structure.jpg/credit}}<br/>|}}]] | |||
|width=250px | |||
|molname=flucloxacillin | |||
|synonyms= see below | |||
|molformula= C<sub>19</sub>H<sub>17</sub>ClFN<sub>3</sub>O<sub>5</sub>S | |||
|molmass= 453.8718 | |||
|uses=antibiotic drug | |||
|properties=beta-lactam | |||
|hazards=see drug interactions | |||
|iupac= see chemistry section | |||
|casnumber=5250-39-5 | |||
}} | |||
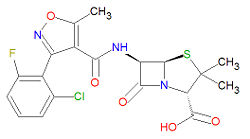
'''Flucloxacillin''' is a [[pencillin]]-like beta-[[lactam]] [[antibiotic]] that is an analog of [[cloxacillin]]. It is used to treat bacterial infections, mostly from aerobic | '''Flucloxacillin''' is a [[pencillin]]-like beta-[[lactam]] [[antibiotic]] that is an analog of [[cloxacillin]]. It is used to treat bacterial infections, mostly from aerobic Gram-positive bacteria. Flucloxacillin, and all penicillin-like drugs, may cause anaphylactic shock. | ||
== Mechanism of action == | == Mechanism of action == | ||
| Line 9: | Line 25: | ||
== Chemistry == | == Chemistry == | ||
Its IUPAC chemical name is (2S,5R,6R)-6-[[3-(2-chloro-6-fluorophenyl)-5-methyl1,2-oxazole-4-carbonyl]amino]-3, | Its IUPAC chemical name is (2S,5R,6R)-6-[[3-(2-chloro-6-fluorophenyl)-5-methyl1,2-oxazole-4-carbonyl]amino]-3, | ||
3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and it has molecular formula C<sub>19</sub>H<sub>17</sub>ClFN<sub>3</sub>O<sub>5</sub>S. Flucloxacillin is stable against degradtion by beta-[[lactamase]]s, including [[penicillinase]]s, and [[cephalosporinase]]s. | 3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and it has molecular formula C<sub>19</sub>H<sub>17</sub>ClFN<sub>3</sub>O<sub>5</sub>S, giving it a molecular mass of 453.8718 g/mol. Flucloxacillin is stable against degradtion by beta-[[lactamase]]s, including [[penicillinase]]s, and [[cephalosporinase]]s. | ||
== Drug interactions == | == Drug interactions == | ||
| Line 15: | Line 31: | ||
== Synonyms and brand names == | == Synonyms and brand names == | ||
''Synonyms'' | {{col-begin}} | ||
{{col-break|width=33%}} | |||
'''Synonyms''' | |||
* Flucloxacilina | * Flucloxacilina | ||
* Flucloxacillin | * Flucloxacillin | ||
| Line 22: | Line 40: | ||
* Flucloxacillinum | * Flucloxacillinum | ||
* Floxacillin | * Floxacillin | ||
{{col-break|width=33%}} | |||
''Brand names'' | '''Brand names''' | ||
* Floxapen | * Floxapen | ||
*Fluclox | *Fluclox | ||
*Sesamol | *Sesamol | ||
{{col-break|width=33%}} | |||
{{col-end}} | |||
== External links == | == External links == | ||
* {{DailyMed}} | * {{DailyMed}} | ||
* {{MedMaster}} | * {{MedMaster}} | ||
* {{DrugBank}} | * {{DrugBank}} | ||
Revision as of 14:01, 3 April 2008
|
| |||||||
| flucloxacillin | |||||||
| |||||||
| Uses: | antibiotic drug | ||||||
| Properties: | beta-lactam | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Flucloxacillin is a pencillin-like beta-lactam antibiotic that is an analog of cloxacillin. It is used to treat bacterial infections, mostly from aerobic Gram-positive bacteria. Flucloxacillin, and all penicillin-like drugs, may cause anaphylactic shock.
Mechanism of action
Like other penicillin drugs, it inhibits the last stage of bacterial cell wall synthesis by binding to penicillin binding proteins, which inhibits the cross-linking needed to form stable cell walls. This leads to autolysis of the bacteria by autolysins.
Chemistry
Its IUPAC chemical name is (2S,5R,6R)-6-[[3-(2-chloro-6-fluorophenyl)-5-methyl1,2-oxazole-4-carbonyl]amino]-3, 3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid and it has molecular formula C19H17ClFN3O5S, giving it a molecular mass of 453.8718 g/mol. Flucloxacillin is stable against degradtion by beta-lactamases, including penicillinases, and cephalosporinases.
Drug interactions
Several drugs, including demeclocycline, doxyclycline, methacycline, minocycline. oxytetracycline, rolitetracycline and tetracycline may be antagonists of flucloxacillin. Flucloxacillin may decrease the effects of the contraceptive drugs ethinyl estradiol and mestranol.
Synonyms and brand names
|
Synonyms
|
Brand names
|
External links
- Flucloxacillin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank