imported>Paul Wormer |
imported>Paul Wormer |
| (51 intermediate revisions by the same user not shown) |
| Line 1: |
Line 1: |
| '''Entropy''' is a function of the state of a [[thermodynamics|thermodynamic system]]. It is a size-extensive<ref>A size-extensive property of a system becomes ''x'' times larger when the system is enlarged by a factor ''x'', provided all intensive parameters remain the same upon the enlargement. Intensive parameters, like temperature and pressure, are independent of size.</ref> quantity with dimension energy divided by temperature ([[SI]] unit: [[joule]]/K). Entropy has no clear analogous mechanical meaning—unlike volume, a similar size-extensive state parameter with dimension energy divided by pressure. Moreover entropy cannot directly be measured, there is no such thing as an entropy meter, whereas state parameters as volume and temperature are easily determined. Consequently entropy is one of the least understood concepts in physics.<ref>It is reported that in a conversation with Claude Shannon, John von Neumann has said: "In the second place, and more important, nobody knows what entropy really is [..]”. M. Tribus, E. C. McIrvine, ''Energy and information'', Scientific American, vol. '''224''' (September 1971), pp. 178–184.</ref>
| | ==Parabolic mirror== |
| {{Image|Carnot title page.jpg|right|300px}} | | {{Image|Refl parab.png|right|350px|Fig. 2. Reflection in a parabolic mirror}} |
| The state variable "entropy" was introduced by [[Rudolf Clausius]] in 1865 when he gave a mathematical formulation of the [[second law of thermodynamics]]. He coined its name from the classical Greek ἐν + τροπή (en = in, at; tropè = change, transformation). On purpose he chose a term similar to "energy", because of the close relationship between the two concepts.
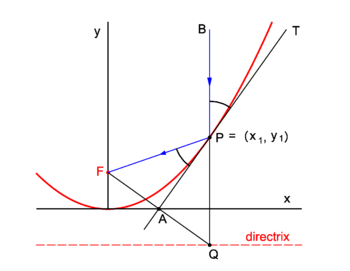
| | Parabolic mirrors concentrate incoming vertical light beams in their focus. We show this. |
|
| |
|
| The traditional way of introducing entropy is by means of a Carnot engine, an abstract engine conceived by [[Sadi Carnot]] (1824)<ref>S. Carnot, ''Réflexions sur la puissance motrice du feu et sur les machines propres à développer cette puissance (Reflections on the motive power of fire and on machines suited to develop that power)'', Chez Bachelier, Paris (1824).</ref> as an idealization of a steam engine. Carnot's work foreshadowed the [[second law of thermodynamics]]. This "engineering" manner of introducing entropy will be discussed below. In this approach, entropy is the amount of heat (per degree kelvin) gained or lost by a thermodynamic system that makes a transition from one state to another.
| | Consider in figure 2 the arbitrary vertical light beam (blue, parallel to the ''y''-axis) that enters the parabola and hits it at point ''P'' = (''x''<sub>1</sub>, ''y''<sub>1</sub>). The parabola (red) has focus in point ''F''. The incoming beam is reflected at ''P'' obeying the well-known law: incidence angle is angle of reflection. The angles involved are with the line ''APT'' which is tangent to the parabola at point ''P''. It will be shown that the reflected beam passes through ''F''. |
|
| |
|
| In the 1870s, [[Ludwig Boltzmann]] gave a definition of entropy in the context of the kinetic gas theory, a branch of physics that developed into statistical thermodynamics. Boltzmann's definition of entropy was furthered by [[John von Neumann]]<ref>Johann von Neumann, ''Mathematische Grundlagen der Quantenmechnik'', Springer, Berlin (1932)</ref> to a quantum statistical definition. This point of view will also be discussed in the present article. In the statistical approach the entropy of an isolated (constant energy) system is ''k''<sub>B</sub>ln''P'', where ''k''<sub>B</sub> is [[Boltzmann]]'s constant, ''P'' is the number of different wave functions of the system belonging to the system's energy (''P'' is the degree of degeneracy, the probability that the state is described by one of the ''P'' wave functions), and the function ln stands for the natural (base ''e'') [[logarithm]].
| | Clearly ∠''BPT'' = ∠''QPA'' (they are vertically opposite angles). Further ∠''APQ'' = ∠''FPA'' because the triangles ''FPA'' and ''QPA'' are congruent and hence ∠''FPA'' = ∠''BPT''. |
|
| |
|
| Not satisfied with the engineering type of argument, the mathematician [[Constantin Carathéodory]] gave in 1909 a new axiomatic formulation of entropy and the second law.<ref>C. Carathéodory, ''Untersuchungen über die Grundlagen der Thermodynamik'' [Investigation on the foundations of thermodynamics], Mathematische Annalen, vol. '''67''', pp. 355-386 (1909).</ref> This was based on [[Pfaffian differential equations]]. His axiom replaced the earlier Kelvin-Planck and the equivalent Clausius formulation of the second law and his method did not invoke Carnot engines. Caratheodory's work was taken up by [[Max Born]],<ref>Physikalische Zeitschrift, vol. 22, p. 218, 249, 282 (1922)</ref> and it reached some textbooks.<ref>H. B. Callen, ''Thermodynamics and an Introduction to Thermostatistics.'' John Wiley and Sons, New York, 2nd edition, (1965); E. A. Guggenheim, ''Thermodynamics'', North-Holland, Amsterdam, 5th edition (1967)</ref> Since it requires more mathematical knowledge than the traditional approach based on Carnot engines, and since this mathematical knowledge is not needed by most students of thermodynamics, the traditional approach is still dominant in the majority of textbooks.
| | We prove the congruence of the triangles: By the definition of the parabola the line segments ''FP'' and ''QP'' are of equal length, because the length of the latter segment is the distance of ''P'' to the directrix and the length of ''FP'' is the distance of ''P'' to the focus. The point ''F'' has the coordinates (0,''f'') and the point ''Q'' has the coordinates (''x''<sub>1</sub>, −''f''). The line segment ''FQ'' has the equation |
| | |
| ==Definition==
| |
| | |
| The state of a [[thermodynamic system]] (a point in state space) is characterized by a number of variables, such as [[pressure]] ''p'', [[temperature]] ''T'', amount of substance ''n'', volume ''V'', etc. Any thermodynamic parameter can be seen as a function of an arbitrary independent set of other thermodynamic variables, hence the terms "property", "parameter", "variable" and "function" are used interchangeably. The number of ''independent'' thermodynamic variables of a system is equal to the number of energy contacts of the system with its surroundings.
| |
| | |
| An example of a reversible (quasi-static) energy contact is offered by the prototype thermodynamical system, a gas-filled cylinder with piston. Such a cylinder can perform work on its surroundings,
| |
| :<math>
| |
| DW = pdV, \quad dV > 0,
| |
| </math>
| |
| where ''dV'' stands for a small increment of the volume ''V'' of the cylinder, ''p'' is the pressure inside the cylinder and ''DW'' stands for a small amount of work. Work by expansion is a form of energy contact between the cylinder and its surroundings. This process can be reverted, the volume of the cylinder can be decreased, the gas is compressed and the surroundings perform work ''DW'' = ''pdV'' ''on'' the cylinder.
| |
| | |
| The small amount of work is indicated by ''D'', and not by ''d'', because ''DW'' is not necessarily a differential of a function. However, when we divide ''DW'' by ''p'' the quantity ''DW''/''p'' becomes obviously equal to the differential ''dV'' of the differentiable state function ''V''. State functions depend only on the actual values of the thermodynamic parameters (they are local), and ''not'' on the path along which the state was reached (the history of the state). Mathematically this means that integration from point 1 to point 2 along path I in state space is equal to integration along a different path II,
| |
| :<math>
| |
| V_2 - V_1 = {\int\limits_1\limits^2}_{{\!\!}^{(I)}} dV
| |
| = {\int\limits_1\limits^2}_{{\!\!}^{(II)}} dV
| |
| \;\Longrightarrow\; {\int\limits_1\limits^2}_{{\!\!}^{(I)}} \frac{DW}{p} =
| |
| {\int\limits_1\limits^2}_{{\!\!}^{(II)}} \frac{DW}{p}
| |
| </math>
| |
| The amount of work (divided by ''p'') performed along path I is equal to the amount of work (divided by ''p'') along path II. This condition is necessary and sufficient that ''DW''/''p'' is a differentiable state function. So, although ''DW'' is not a differential, the quotient ''DW''/''p'' is one.
| |
| | |
| Reversible absorption of a small amount of heat ''DQ'' is another energy contact of a system with its surroundings; ''DQ'' is again not a differential of a certain function. In a completely analogous manner to ''DW''/''p'', the following result can be shown for the heat ''DQ'' (divided by ''T'') absorbed by the system along two different paths (along both paths the absorption is reversible):
| |
| | |
| <div style="text-align: right;" > | |
| <div style="float: left; margin-left: 35px;" >
| |
| <math>{\int\limits_1\limits^2}_{{\!\!}^{(I)}}\frac{DQ}{T} = {\int\limits_1\limits^2}_{{\!\!}^{(II)}} \frac{DQ}{T} .
| |
| </math>
| |
| </div>
| |
| <span id="(1)" style="margin-right: 200px; vertical-align: -40px; ">(1)</span>
| |
| </div>
| |
| <br><br>
| |
| Hence the quantity ''dS'' defined by
| |
| :<math>
| |
| dS \;\stackrel{\mathrm{def}}{=}\; \frac{DQ}{T}
| |
| </math>
| |
| is the differential of a state variable ''S'', the ''entropy'' of the system. In a later subsection equation (1) will be proved from the Clausius/Kelvin principle. Observe that this definition of entropy only fixes entropy differences:
| |
| :<math> | | :<math> |
| S_2-S_1 \equiv \int_1^2 dS = \int_1^2 \frac{DQ}{T}
| | \lambda\begin{pmatrix}0\\ f\end{pmatrix} + (1-\lambda)\begin{pmatrix}x_1\\ -f\end{pmatrix}, \quad 0\le\lambda\le 1. |
| </math> | | </math> |
| Note further that entropy has the dimension energy per degree temperature (joule per degree kelvin) and recalling the [[first law of thermodynamics]] (the differential ''dU'' of the [[internal energy]] satisfies ''dU'' = ''DQ'' − ''DW''), it follows that
| | The midpoint ''A'' of ''FQ'' has coordinates (λ = ½): |
| :<math>
| |
| dU = TdS - pdV.\,
| |
| </math>
| |
| (For convenience sake only a single work term was considered here, namely ''DW'' = ''pdV'', work done ''by'' the system).
| |
| The internal energy is an extensive quantity, that is, when the system is doubled, ''U'' is doubled too. The temperature ''T'' is an intensive property, independent of the size of the system. The entropy ''S'', then, is an extensive property. In that sense the entropy resembles the volume of the system.
| |
| | |
| An important difference between ''V'' and ''S'' is that the former is a state function with a well-defined mechanical meaning, whereas entropy is introduced by analogy and is not easily visualized. Indeed, as is shown in the next subsection, it requires a fairly elaborate reasoning to prove that ''S'' is a state function, i.e., equation [[#(1)|(1)]] to hold.
| |
| | |
| | |
| | |
| | |
| ===Proof that entropy is a state function===
| |
| When equation [[#(1)|(1)]] has been proven, the entropy ''S'' is shown to be a state function. The standard proof, as given now, is physical, by means of [[Carnot cycle]]s, and is based on the Clausius/Kelvin formulation of the second law given in the introduction.
| |
| {{Image|Entropy.png|right|350px|Fig. 1. ''T'' > ''T''<sub>0</sub>. (I): Carnot engine E moves heat from heat reservoir R to "condensor" C and needs input of work DW<sub>in</sub>. (II): E generates work DW<sub>out</sub> from the heat flow from C to R. }} An alternative, more mathematical proof, postulates the existence of a state variable ''S'' with certain properties and derives the existence of [[thermodynamical temperature]] and the second law from these properties.
| |
| | |
| In figure 1 a finite heat bath C ("condensor")<ref>Because of a certain similarity of C with the condensor of a steam engine C is referred as "condensor". The quotes are used to remind us that nothing condenses, unlike the steam engine where steam condenses to water</ref> of constant volume and variable temperature ''T'' is shown. It is connected to an infinite heat reservoir R through a reversible Carnot engine E. Because R is infinite its temperature ''T''<sub>0</sub> is constant, addition or extraction of heat does not change ''T''<sub>0</sub>. It is assumed that always ''T'' ≥ ''T''<sub>0</sub>. One may think of the system E-plus-C as a ship and the heat reservoir R as the sea. The following argument then deals with an attempt of extracting energy from the sea in order to move the ship, i.e., with an attempt to let E perform net outgoing work in a cyclic (i.e., along a closed path in the state space of C) process.
| |
| | |
| A Carnot engine performs reversible cycles (in the state space of E, not be confused with cycles in the state space of C) and per cycle either generates work ''DW''<sub>out</sub> when heat is transported from high temperature to low temperature (II), or needs work ''DW''<sub>in</sub> when heat is transported from low to high temperature (I), in accordance with the Clausius/Kelvin formulation of the second law.
| |
| | |
| The definition of [[thermodynamical temperature]] (a positive quantity) is such that for II,
| |
| :<math>
| |
| \frac{DW_\mathrm{out}}{DQ} = \frac{T-T_0}{T},
| |
| </math>
| |
| while for I
| |
| :<math>
| |
| \frac{DW_\mathrm{in}}{DQ_0} = \frac{T-T_0}{T_0}.
| |
| </math>
| |
| | |
| The first law of thermodynamics states for I and II, respectively,
| |
| :<math>
| |
| -DW_\mathrm{in} -DQ_0 + DQ=0\quad\hbox{and}\quad DW_\mathrm{out} + DQ_0-DQ=0
| |
| </math>
| |
| {{Image|Cycle entropy.png|right|150px|Fig. 1. Two paths in the state space of the "condensor" C.}}
| |
| For I,
| |
| :<math>
| |
| \begin{align}
| |
| \frac{DW_\mathrm{in}}{DQ_0} &= \frac{DQ- DQ_0}{DQ_0} = \frac{DQ}{DQ_0} -1 \\
| |
| &=\frac{T-T_0}{T_0} = \frac{T}{T_0} - 1 \;
| |
| \Longrightarrow DQ_0 = T_0 \left(\frac{DQ}{T}\right)
| |
| \end{align}
| |
| </math>
| |
| | |
| For II we find the same result,
| |
| :<math>
| |
| \begin{align}
| |
| \frac{DW_\mathrm{out}}{DQ} &= \frac{DQ- DQ_0}{DQ} = 1- \frac{DQ_0}{DQ} \\
| |
| &=\frac{T-T_0}{T} = 1- \frac{T_0}{T}
| |
| \;\Longrightarrow DQ_0 = T_0 \left(\frac{DQ}{T}\right)
| |
| \end{align}
| |
| </math>
| |
| In figure 2 the state diagram of the "condensor" C is shown. Along path I the Carnot engine needs input of work to transport heat from the colder reservoir R to the hotter C and the absorption of heat by C raises its temperature and pressure. Integration of ''DW''<sub>in</sub> = ''DQ'' − ''DQ''<sub>0</sub> (that is, summation over many cycles of the engine E) along path I gives
| |
| :<math>
| |
| W_\mathrm{in} = Q_\mathrm{in} - T_0 {\int\limits_1\limits^2}_{{\!\!}^{(I)}} \frac{DQ}{T} \quad\hbox{with}\quad Q_\mathrm{in} \equiv {\int\limits_1\limits^2}_{{\!\!}^{(I)}} DQ.
| |
| </math>
| |
| Along path II the Carnot engine delivers work while transporting heat from C to R. Integration of ''DW''<sub>out</sub> = ''DQ'' − ''DQ''<sub>0</sub> along path II gives
| |
| :<math> | |
| W_\mathrm{out} = Q_\mathrm{out} - T_0 {\int\limits_2\limits^1}_{{\!\!}^{(II)}} \frac{DQ}{T}
| |
| \quad\hbox{with}\quad Q_\mathrm{out} \equiv {\int\limits_2\limits^1}_{{\!\!}^{(II)}} DQ
| |
| </math>
| |
| | |
| Assume now that the amount of heat ''Q''<sub>out</sub> extracted (along path II) from C and the heat ''Q''<sub>in</sub> delivered (along I) to C are the same in absolute value. In other words, after having gone along a closed path in the state diagram of figure 2, the condensor C has not gained or lost heat. That is,
| |
| :<math> | | :<math> |
| Q_\mathrm{in} + Q_\mathrm{out} = 0, \,
| | \frac{1}{2}\begin{pmatrix}0\\ f\end{pmatrix} + \frac{1}{2}\begin{pmatrix}x_1\\ -f\end{pmatrix} = |
| | \begin{pmatrix}\frac{1}{2} x_1\\ 0\end{pmatrix}. |
| </math> | | </math> |
| then
| | Hence ''A'' lies on the ''x''-axis. |
| | The parabola has equation, |
| :<math> | | :<math> |
| W_\mathrm{in} + W_\mathrm{out} = - T_0 {\int\limits_1\limits^2}_{{\!\!}^{(I)}} \frac{DQ}{T}
| | y = \frac{1}{4f} x^2. |
| - T_0 {\int\limits_2\limits^1}_{{\!\!}^{(II)}} \frac{DQ}{T}.
| |
| </math> | | </math> |
| If the total net work ''W''<sub>in</sub> + ''W''<sub>out</sub> is positive (outgoing), this work is done by heat obtained from R, which is not possible because of the Clausius/Kelvin principle. If the total net work ''W''<sub>in</sub> + ''W''<sub>out</sub> is negative, then by inverting all reversible processes, i.e., by going down path I and going up along II, the net work changes sign and becomes positive (outgoing). Again the Clausius/Kelvin principle is violated. The conclusion is that the net work is zero and that
| | The equation of the tangent at ''P'' is |
| :<math> | | :<math> |
| T_0 {\int\limits_1\limits^2}_{{\!\!}^{(I)}} \frac{DQ}{T} +
| | y = y_1 + \frac{x_1}{2f} (x-x_1)\quad \hbox{with}\quad y_1 = \frac{x_1^2}{4f}. |
| T_0 {\int\limits_2\limits^1}_{{\!\!}^{(II)}} \frac{DQ}{T} = 0
| |
| \;\Longrightarrow\; {\int\limits_1\limits^2}_{{\!\!}^{(I)}} \frac{DQ}{T} = {\int\limits_1\limits^2}_{{\!\!}^{(II)}} \frac{DQ}{T}. | |
| </math> | | </math> |
| From this independence of path it is concluded that
| | This line intersects the ''x''-axis at ''y'' = 0, |
| :<math> | | :<math> |
| dS \equiv \frac{DQ}{T}
| | 0 = \frac{x_1^2}{4f} - \frac{x_1^2}{2f} + \frac{x_1}{2f} x |
| | \Longrightarrow \frac{x_1}{2f} x = \frac{x_1^2}{4f} \longrightarrow x = \tfrac{1}{2}x_1. |
| </math> | | </math> |
| is a state (local) variable. | | The intersection of the tangent with the ''x''-axis is the point ''A'' = (½''x''<sub>1</sub>, 0) that lies on the midpoint of ''FQ''. The corresponding sides of the triangles ''FPA'' and ''QPA'' are of equal length and hence the triangles are congruent. |
| ==Footnotes==
| |
| <references /> | |
| ==References ==
| |
| * M. W. Zemansky, ''Kelvin and Caratheodory—A Reconciliation'', American Journal of Physics Vol. '''34''', pp. 914-920 (1966) [http://dx.doi.org/10.1119/1.1972279]
| |