User talk:Paul Wormer/scratchbook1: Difference between revisions
imported>Paul Wormer No edit summary |
imported>Paul Wormer |
||
| Line 23: | Line 23: | ||
Summarizing, | Summarizing, | ||
:<math> | :<math> | ||
\mathcal{E}_\mathrm{X}(q) = \mathcal{E}_\mathrm{Y}(-q)\quad \hbox{and}\quad \mathcal{E}_\mathrm{X}(-q) = \mathcal{E}_\mathrm{Y}(q) | \mathcal{E}_\mathrm{X}(q) = \mathcal{E}_\mathrm{Y}(-q) | ||
\quad | |||
\hbox{and} | |||
\quad | |||
\mathcal{E}_\mathrm{X}(-q) = \mathcal{E}_\mathrm{Y}(q) | |||
</math> | |||
with | |||
:<math> | |||
\mathcal{E}_\mathrm{X}(q) <\mathcal{E}_\mathrm{Y}(q)\quad\hbox{for}\quad q > 0. | |||
</math> | </math> | ||
| Line 30: | Line 38: | ||
The above arguments are not restricted to square molecules. With the exception of linear molecules, which show [[Renner-Teller effect]]s, all polyatomic molecules of sufficiently high symmetry to possess spatially degenerate electronic states will be subject to the Jahn-Teller instability. The proof, as given by Jahn and Teller and reproduced in the next section, proceeds by application of point group symmetry principles; it rests on inspection of all molecular symmetry groups | The above arguments are not restricted to square molecules. With the exception of linear molecules, which show [[Renner-Teller effect]]s, all polyatomic molecules of sufficiently high symmetry to possess spatially degenerate electronic states will be subject to the Jahn-Teller instability. The proof, as given by Jahn and Teller and reproduced in the next section, proceeds by application of point group symmetry principles; it rests on inspection of all molecular symmetry groups | ||
<br><br> | <br><br> | ||
==Jahn-Teller proof== | ==Jahn-Teller proof== | ||
The proof given by Jahn and Teller in 1937 is based on first-order [[perturbation theory]] and application of [[point group]] symmetry. | The proof given by Jahn and Teller in 1937 is based on first-order [[perturbation theory]] and application of [[point group]] symmetry. | ||
Revision as of 09:23, 22 February 2010
In molecular physics, the Jahn-Teller effect is the distortion of a highly symmetric—but non-linear—molecule to lower symmetry and lower energy. The effect occurs if the high-symmetric molecule would be in a degenerate state of definite energy, that is, if more than one wave function of the same energy would be eigenfunction of the molecular Hamiltonian. When energy degeneracy of a molecular state would arise—i.e., two or more orthogonal wave functions would describe the same molecular-energy eigenstate—it appears that a distorted, lower-symmetry, molecule will have lower, and accordingly more favorable, energy. Hence, by Jahn-Teller distortion, the molecule is lowered in symmetry and the energy degeneracy is lifted. Wave functions that would belong to the same energy in the high-symmetric molecule obtain different energies, some energies are lower, some higher.
The effect is named after H. A. Jahn and E. Teller who predicted it in 1937.[1] It took some time before the effect was experimentally observed, because it was masked by other molecular interactions. However, there are now numerous unambiguous observations that agree well with theoretical predictions. These range from the excited states of the simplest non-linear molecule H3, through moderate sized organic molecules, like cations of substituted benzene, to transition metal complexes and localized impurity centers in solids.
- The Jahn-Teller effect has a quantum mechanical origin and there is no classical-physics explanation of it. Therefore, some knowledge of quantum mechanics is prerequisite to the reading of this article. Further Schönflies notation for point groups and Mulliken notation for their irreducible representations is used without further explanation.
Explanation

Fig. 1 The middle molecule at the top is square planar (point group D4h). The red arrows indicate a stretch-type normal mode and the rhombus-shaped molecules on either side are obtained by deforming the middle molecule along the normal mode. The degenerate electronic energy is split by the deformation as shown at the bottom.
The Jahn-Teller effect is best explained by an example. Consider to that end the square homonuclear molecule in the middle of the top row of Fig. 1. The plane of the figure is the xy-plane, with the y-axis vertical and the x-axis horizontal. The z-axis points towards the reader. The symmetry group of the middle molecule is D4h. Let there be two—in principle exact—electronic wave functions that together span the irreducible representation Eu of this group. One wave function transforms as an x-coordinate under D4h and is denoted by |X ⟩. Its partner transforms as a y-coordinate and is denoted by |Y ⟩. In the case of the perfect square both wave functions have the same energy denoted by .
It may be helpful to mention that approximations for the wave functions can be obtained from two degenerate molecular orbitals |x ⟩ and |y ⟩ (together carrying Eu) that are outside a closed-shell and share a single unpaired electron. A closed-shell wave function (a many-electron wave function consisting of doubly occupied molecular orbitals) is invariant under the group operations. The exact wave function |X ⟩ is then approximated by the closed-shell wave function times orbital |x ⟩ containing the single electron, while |Y ⟩ is approximated by the closed-shell wave function times molecular orbital |y⟩ containing the electron. The orbital |x ⟩ transforming as an x-coordinate has the yz-plane—a mirror plane—as a nodal plane, that is, the orbital vanishes in this plane and has plus sign to the right and minus sign to the left of the yz-plane. Similarly the orbital |y ⟩ has the xz-plane as a nodal plane.
In the middle molecule shown in Fig. 1 an in-plane vibrational stretching normal mode Q of the planar molecule is indicated by red arrows. Explicitly, with respect to local, parallel, right-handed systems of axes, the red arrows represent the mode
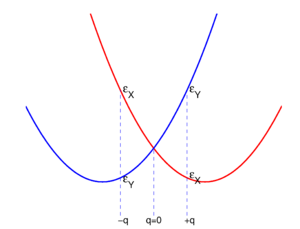
where the deviations of the atoms are all of the same length |q|.[2] When q is positive, the molecule is elongated along the y-axis and compressed along the x-axis; this is the rightmost molecule in Fig. 1. Similarly, negative q implies a compression along the y-axis and an elongation along the x-axis (the leftmost molecule). The case q = 0 corresponds to the perfect square.
Both distorted rhombus-shaped molecules are of D2h symmetry; D2h is an Abelian group that has—as any Abelian group—only one-dimensional irreducible representations. Hence all electronic states of the distorted molecules are non-degenerate. The function |X ⟩ transforms as B3u and |Y ⟩ transforms as B2u of D2h. Let the respective energies be written as and . Group theory tells us that these energies are different, but without explicit calculation it is not a priori clear which of the two energies is higher. Let us assume that for positive q : . This is shown in the energy level scheme in the bottom part of Fig. 1. An important observation now is that the leftmost and rightmost distorted molecules are essentially the same, they follow from each other by an in-plane rotation over ±90° around the z-axis. The x and y direction are interchanged between the left- and rightmost molecules by this rotation. Hence the molecule distorted with negative q-value has the energies satisfying: .
Summarizing,
with
For small q-values it is reasonable to assume that both and are quadratic functions of q. The quadratic energy curves are shown in Fig. 2. For q = 0 the energy curves cross and . The crossing point, corresponding to the perfect square, is clearly not an absolute minimum; therefore, the totally square symmetric configuration of the molecule will not be a stable equilibrium for the degenerate electronic state. At equilibrium, the molecule will be distorted along a normal mode away from square, and its energy will be lowered. This is the Jahn-Teller effect.
The above arguments are not restricted to square molecules. With the exception of linear molecules, which show Renner-Teller effects, all polyatomic molecules of sufficiently high symmetry to possess spatially degenerate electronic states will be subject to the Jahn-Teller instability. The proof, as given by Jahn and Teller and reproduced in the next section, proceeds by application of point group symmetry principles; it rests on inspection of all molecular symmetry groups
Jahn-Teller proof
The proof given by Jahn and Teller in 1937 is based on first-order perturbation theory and application of point group symmetry.
Let us write r for the electronic coordinates and R for the nuclear coordinates of a molecule. The highly symmetric configuration (the one that is deformed by the Jahn-Teller effect) is designated by R0. The clamped nuclei Hamiltonian that arises in the first step of the Born-Oppenheimer approximation is
where ΔR is a small deformation of the nuclear framework away from the symmetric case. The displacements are assumed to be infinitesimally small, i.e., |ΔR|2 << |ΔR|. This means that the expansion of H can be truncated after the second term, but also that a first-order perturbation approximation is valid; higher-order approximations contain terms non-linear in the displacements.
The first term on the right-hand side in the previous expression is the zeroth-order Hamiltonian, which by assumption has a degenerate set of electronic eigenstates spanning an irreducible representation μ of the point group of the (undeformed) molecule:
where fμ is the dimension of μ (the degree of degeneracy of ). The second term is the perturbation
In accordance with first-order perturbation theory the electronic energies of the slightly deformed molecule are given by
where the first-order energies are the eigenvalues of the fμ×fμ matrix with general element
and the angular brackets imply integration over the electronic coordinates r.
In order to predict whether these matrix elements are vanishing, Jahn and Teller transformed the nuclear Cartesian displacement coordinates ΔRAα to symmetry adapted coordinates Qk by a linear non-singular transformation L
where A runs over the Nnuc nuclei of the molecule and α over the Cartesian coordinates of RA. The elements of the matrix L can be determined by group theoretical projection (an example of a symmetry coordinate Q was given in the previous section; the example specifies one of the twelve rows of L). Jahn and Teller tabulate the irreducible representations carried by the nuclear coordinates for all possible point groups. They consider also the overall rotation and translation of the molecules.
For instance, they list that for the group D4h (the group of four nuclei on the corners of a square) the components of the translation vector span A2u and E1u. The rotation generators (components of the rigid rotor angular momentum) span A2g and E1g, and the internal (vibrational) coordinates span A1g, B1g, B2g, B2u, and Eu. The twelve rows of the matrix L are labeled by these irreducible representations (and in the case of a more-dimensional representation μ by a further subindex running from 1 to to fμ).
By a simple application of the chain rule it follows that
where it is assumed that the molecule moves in a homogeneous and isotropic space so that V does not depend on the 3 translational and 3 rotational symmetry coordinates. For the groups of interest ∂/∂Qk transforms as Qk and hence Vk(r) is an operator that transforms according to the same irreducible representation as Qk. It is a multiplicative operator, i.e.,
since the electronic kinetic energy terms are contained in the zeroth-order Hamiltonian H(r, R0). The groups of interest all have real irreducible representation matrices, which implies that the zeroth-order wave functions are real.
The product spans the symmetrized representation [μ2], which is of dimension fμ(fμ + 1)/2 and reducible if fμ > 1. Jahn and Teller tabulate the decompositions of all symmetrized representations of all pertinent groups in irreducible components. For instance for D4h:
Group theory tells us that a perturbation matrix element
unless the irreducible representation of Vk is contained in the decomposition of [μ2]. By inspection of their tables Jahn and Teller found that all symmetry groups of all non-linear molecules have at least one (but usually more) non-vanishing perturbation matrix elements.
For instance, assume that a molecule with D4h symmetry has a zeroth-order electronic state belonging to Eu. One needs to inspect
where the first factor corresponds to V and the second to [Eu2]. Non-vanishing cases are A1g⊗A1g and B1g⊗B1g
Say a matrix element of Vk ≠ 0. Then the corresponding displacement Qk appears linearly in the lowest first-order energy correction (lowest eigenvalue of the perturbation matrix); terms of higher order in the infinitesimal coordinate Qk can be ignored. Suppose this lowest first-order energy correction contains CQk with some real constant C. If C > 0 then a displacement along −Qk lowers the electronic energy and the molecule will deform in that direction. If C < 0 then a displacement along Qk decreases the electronic energy. The case C = 0 only arises when the whole perturbation matrix is zero and this case is excluded.
In the previous example for D4h, the case A1g⊗A1g corresponds to a symmetric stretch that does not lower the symmetry of the molecule. During this "breathing" vibration the molecule alternately expands and shrinks, but keeps its shape. The other non-zero case is B1g⊗B1g. This corresponds to a deformation of the molecule along a coordinate Q of B1g symmetry. This corresponds to a deformation of the square molecule to a rhombus-shaped molecule, as was shown in the previous section.
Reference
- R. Englman, The Jahn-Teller Effect in Molecules and Crystals, Wiley, London (1972). ISBN-10: 0471241687 ISBN-13: 78-0471241683
- Isaac B. Bersuker, The Jahn-Teller Effect, Cambridge University Press (2006). ISBN-10: 0521822122 ISBN-13: 9780521822121






















![{\displaystyle [\mathrm {E} _{g}^{2}]=[\mathrm {E} _{u}^{2}]=\mathrm {A} _{1g}\oplus \mathrm {B} _{1g}\oplus \mathrm {B} _{2g}.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5eb1764d9dc12aeab5db98b669d49a4f7db5ffd8)

