Taurine: Difference between revisions
imported>David E. Volk No edit summary |
imported>David E. Volk (Biosynthesis diagram and discussion) |
||
| Line 3: | Line 3: | ||
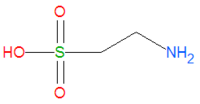
'''Taurine''', or '''2-aminoethanesulfonic acid''', is a natural biochemical produced by many, but not all, animals, and is a major constituent of [[bile]]. It is often referred to as an [[amino acid]] despite the fact that it does not contain a [[carboxylic acid]] group like most amino acids do. It plays an important role in the phase II detoxification of the liver by forming water soluble salts which can be excreted. Chemically, it is one of the few naturally occuring [[sulfonic acid]]s and it a derivative of [[cysteine]], another amino acid important in liver detoxification. | '''Taurine''', or '''2-aminoethanesulfonic acid''', is a natural biochemical produced by many, but not all, animals, and is a major constituent of [[bile]]. It is often referred to as an [[amino acid]] despite the fact that it does not contain a [[carboxylic acid]] group like most amino acids do. It plays an important role in the phase II detoxification of the liver by forming water soluble salts which can be excreted. Chemically, it is one of the few naturally occuring [[sulfonic acid]]s and it a derivative of [[cysteine]], another amino acid important in liver detoxification. | ||
== Biosynthesis == | |||
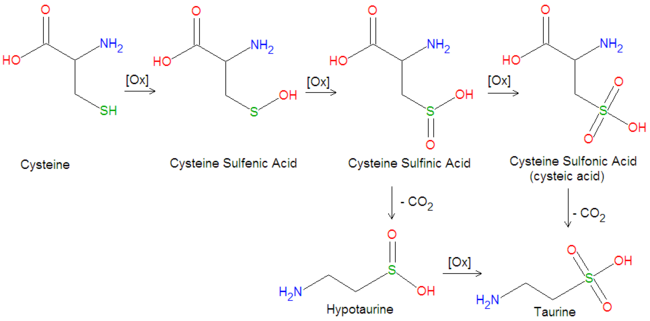
{{Image|Taurine biosynthesis.png|center|650px|Biosynthetic conversion of cysteine into taurine.}} | |||
Taurine is created enzymatically from L-[[cysteine]] by several oxidation reactions and a single decarboxylation step, as illustated in the figure. In the first oxidation step, the hydrogen on the sulfur atom is replaced with an [[hydroxyl]] group (OH), creating a [[sulfenic acid]], [[cysteine sulfenic acid]]. The second oxidation adds another oxygen atom to produce a [[sulfinic acid]], [[cysteine sulfinic acid]]. A third oxidation step adds another oxygen atom to form a [[sulfonic acid]], [[cysteine sulfonic acid]], also called cysteic acid. An enzymatic [[decarboxylation]] reaction, in which [[carbon dioxide]] (CO<sub>2</sub>) is removed, produces taurine. In an alternative pathway, the decarboxylation step may precede the final oxidation. Thus, decarboxylation of cystein sulfinic acid produces [[hypocysteine]], which can be subsequently oxidized to form taurine. | |||
Revision as of 09:29, 6 October 2009
Taurine, or 2-aminoethanesulfonic acid, is a natural biochemical produced by many, but not all, animals, and is a major constituent of bile. It is often referred to as an amino acid despite the fact that it does not contain a carboxylic acid group like most amino acids do. It plays an important role in the phase II detoxification of the liver by forming water soluble salts which can be excreted. Chemically, it is one of the few naturally occuring sulfonic acids and it a derivative of cysteine, another amino acid important in liver detoxification.
Biosynthesis
Taurine is created enzymatically from L-cysteine by several oxidation reactions and a single decarboxylation step, as illustated in the figure. In the first oxidation step, the hydrogen on the sulfur atom is replaced with an hydroxyl group (OH), creating a sulfenic acid, cysteine sulfenic acid. The second oxidation adds another oxygen atom to produce a sulfinic acid, cysteine sulfinic acid. A third oxidation step adds another oxygen atom to form a sulfonic acid, cysteine sulfonic acid, also called cysteic acid. An enzymatic decarboxylation reaction, in which carbon dioxide (CO2) is removed, produces taurine. In an alternative pathway, the decarboxylation step may precede the final oxidation. Thus, decarboxylation of cystein sulfinic acid produces hypocysteine, which can be subsequently oxidized to form taurine.