Liquid extraction: Difference between revisions
imported>Lisa Lema |
imported>Lisa Lema |
||
| Line 35: | Line 35: | ||
<ref>Miereles, Angela, Extracting Bioactive Compounds for Food Products, Boca Raton: CRC Press, 2009. </ref> | <ref>Miereles, Angela, Extracting Bioactive Compounds for Food Products, Boca Raton: CRC Press, 2009. </ref> | ||
====SuperCritical Extraction==== | =====SuperCritical Extraction===== | ||
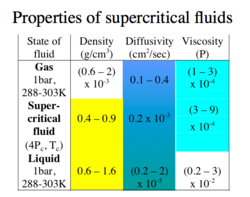
Supercritical extractions are possible with the use of supercritical fluids. A supercritical fluid has a pressure and temperature above its critical point. These fluids display unique characteristics that enable then to be used as solvents. The density of these fluids are relatively high, and consequently | Supercritical extractions are possible with the use of supercritical fluids. A supercritical fluid has a pressure and temperature above its [[critical point]]. These fluids display unique characteristics that enable then to be used as[[ solvents]]. The density of these fluids are relatively high, and consequently have high solvation power. | ||
Separation is achieved by decreasing the pressure or increasing the temperature of the mixture that leaves the extraction column. | Separation is achieved by decreasing the pressure or increasing the temperature of the mixture that leaves the extraction column. Another method includes the back-extraction with water or another solvent to improve the quality of the extraction. | ||
Supercritical fluid extraction is used for the decaffeination of coffee and extraction of hops, spcies, flavors, and vegetables. | |||
{{Image|Supercritical Fluid Properties.png|right|250px|.}} | |||
<ref>Treybal, Robert E. Mass Transfer Operations. New York: McGraw-Hill Book Company, 1968.</ref> | |||
<ref>Nahar, Lutfun and Satyajit D. Sarker. "Supercritical Fluid Extraction." Methods in Biotechnology 20 (2005): 47-73. </ref> | |||
====Solvent Selection for Liquid Extraction==== | ====Solvent Selection for Liquid Extraction==== | ||
Revision as of 12:36, 17 December 2010
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is 21 December 2010. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! Note to course participants: Looking forward to some insightful and useful articles from your collaborations. |
Extraction
Solvent Extraction is a process that separates components in a matrix by contact with another liquid. Typical extractions include, liquid-solid extractions, liquid-liquid extractions and Supercritical extractions. Separation is based on the compounds’ physical and chemical properties.
Extraction is widely used in the chemical and biochemical industries. It often is the most efficient method of separation. Some extraction techniques involve partition between two immiscible liquids, others involve either continuous extractions or batch extractions.
Due to environmental concerns some of the solvents used have become environmentally friendly. The process itself have been modified to make use of more environmentally friendly solvents. The solvents could vary from vapor, supercritical fluid, or liquid.
History
Solvent extraction is an old practice done for years. It is the main process in perfume development and it is also used to obtain dyes from various sources.
Modern use has made this process an essential practice in scientific laboratories. In 1872 Berthelot and Jungfleisch introduced the concept of the partition coefficient. Followed by an introduction in phase equilibrium by Gibbs. Many of these advances occurred as the chemical industry grew as well.
Further research and improvements to the process affected the commercial applications. Due to the advantages of solvent extraction various chemical treatment methods for refining coal tar products and mineral oil were replaced.
Design and Operation
Liquid-Liquid Extraction
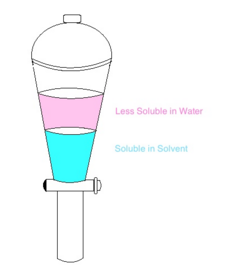
Liquid-liquid extraction is a process for separating the components of a liquid (the feed) by contact with a second liquid phase (the solvent). The process takes advantage of differences in the chemical properties of the feed components, such as differences in polarity and hydrophobic/hydrophilic character, to separate them. The methods vary from simple single-stage extractions to complex multistage extractions.
Single Stage Extraction A simple and small scale procedure common in chemical laboratories. It requires the use of a separating funnel and like the one shown above. This procedure is very simple using a limited amount of solvent.
Multistage Extraction More prevalent in industry, this process feeds the raffinate from one extraction unit to another continuously. This ensures the extraction of the substances.
SuperCritical Extraction
Supercritical extractions are possible with the use of supercritical fluids. A supercritical fluid has a pressure and temperature above its critical point. These fluids display unique characteristics that enable then to be used assolvents. The density of these fluids are relatively high, and consequently have high solvation power.
Separation is achieved by decreasing the pressure or increasing the temperature of the mixture that leaves the extraction column. Another method includes the back-extraction with water or another solvent to improve the quality of the extraction.
Supercritical fluid extraction is used for the decaffeination of coffee and extraction of hops, spcies, flavors, and vegetables.
Solvent Selection for Liquid Extraction
Selectivity: How effective a solvent is dependent on the solution being separated and the concentration of solutes present in the solutions. The ratio of ratios, the separation factor, or selectivity, β, is analogous to the relative volatility of distillation. E and R are equilibrium phases, C and A are solutions being separated. Failed to parse (syntax error): {\displaystyle β= (wt fraction C in E )/(wt fraction A in E)/(wt fraction C in R)/(wt fraction A in R) = }
A good extraction will occur when the selectivity exceeds unity. If selectivity is ever in unity no separation would occur.
Distribution Coefficient: In our example this is the ratio at equilibrium. Less solvent is required for the extraction the larger this ratio is.
Insolubility of Solvent: The solvent must be insoluble to the solution being extracted.
Recoverability: After extraction is completed the solvent must be recovered. This recovery is usually done through distillation, making sure no azeotrope is formed, and mixtures should show high relative volatility for low-cost recovery. Any substance in the extract, either solvent or solute must be present in small concentrations and be more volatile in order to reduce heat costs. If the solvent must be volatized, its latent heat of vaporization should be small.
Density: A difference in densities of the saturated liquid phases is necessary, both for stagewise and continuous-contact equipment operation. The larger this difference the better.
Interfacial Tension: Similar to surface tension in which cohesive forces are involved. However, the main force involved is the adhesive force between the liquid phase of one substance and the phase of another substance. The interaction occurs at the surfaces of the substances involved, is at their interfaces. The larger the interfacial tension the more readily will coalescence of emulsions occur, but the more difficult will the dispersion of one liquid in the other be.
Chemical Reactivity: The solvent should be stable chemically and inert toward the other components of the system and toward the common materials of construction.
Viscosity, vapor pressure, and freezing point:These should be low for ease in handling and storage.
Other: Solvent should be nontoxic, nonflammable, and of low cost.
Applications
This section should discuss how the process is used in practice.[4]
Examples
If you have used a lot of equations in your article, this may be a good place to show an example of how they are used. See the article on the Antoine Equation for an example.
References
- ↑ Miereles, Angela, Extracting Bioactive Compounds for Food Products, Boca Raton: CRC Press, 2009.
- ↑ Treybal, Robert E. Mass Transfer Operations. New York: McGraw-Hill Book Company, 1968.
- ↑ Nahar, Lutfun and Satyajit D. Sarker. "Supercritical Fluid Extraction." Methods in Biotechnology 20 (2005): 47-73.
- ↑ "Major Success for Bioprocess Fractionation," Anytown Daily News, January 1, 2015, p. A6.