Cocaine: Difference between revisions
imported>Richard Pettitt mNo edit summary |
mNo edit summary |
||
| (16 intermediate revisions by 7 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
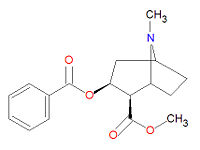
{{Image|Cocaine structure.jpg|right|200px|Cocaine}} | |||
'''Cocaine''' or '''benzoylmethyl ecgonine''' (IUPAC name methyl (2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate) is an organic [[chemistry|chemical]] compound that belongs to the [[alkaloid]] category. Its chemical formula is C<sub>17</sub>H<sub>21</sub>NO<sub>4</sub>. Cocaine is a colorless or white [[crystal]]line substance. It is primarily extracted from [[coca]] plant, largely grown in [[South America]]. | |||
Cocaine is primarily used recreationally for its stimulating and euphoric effects on human body. Due to its abuse, the possession, production and distribution of cocaine has been declared illegal by most [[country|countries]]. In the United States, it is classified by the [[Controlled Substance Act]] as a Schedule II substance, allowing only limited medical use. Along with [[heroin]] and [[methamphetamine]], cocaine is often considered a "[[hard drug]]", being much more physically addictive and harmful than a so-called "[[soft drug]]" like [[marijuana]]. Despite harsh penal codes against the consumption, production, and distribution of the drug, a [[black market]] is still flourishing. Street names for cocaine include coke, snow, and blow, among others. | |||
Cocaine | ==History== | ||
Cocaine was first refined from raw coca in 1860 by Albert Niemann, a German graduate student working under Friedrich Wöhler in Göttingen University. In the decades after its original isolation, cocaine was used as an experimental drug in a wide variety of clinical applications by researchers including [[Sigmund Freud]]. <ref>{{cite book|title=Andean cocaine: the making of a global drug|first=Paul|last=Gootenberg|year=2009|publisher=University of North Carolina Press|location=Chapel Hill|id=ISBN 9780807859056}}</ref> It soon came to be used in a variety of commercial products including some that were very popular, including [[Vin Mariani]] and [[Coca Cola]]. Cocaine production was seen as a significant economic resource and a modernizing export in Peru. It was not until after World War I that U.S. pressure abroad made cocaine an illicit product on the international stage. | |||
== Pharmacology == | == Pharmacology == | ||
The majority of cocaine's effects are due to increasing monoamine levels by binding to [[serotonin]] (5-HT), [[norepinephrine]] (NE), and [[dopamine]] (DA) [[catecholamine plasma membrane transport protein]]s. This blockade prevents the reuptake of neurotransmitters, thus increasing the amount available at the synapse to bind to the post synaptic cell. In contrast to [[amphetamine]], a similar psychomotor stimulant, cocaine is a [[dopamine uptake inhibitor]] and does not stimulate DA release.<ref>{{cite journal |author=Gold LH, Geyer MA, Koob GF |title=Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs |journal=NIDA Res. Monogr. |volume=94 |issue= |pages=101–26 |year=1989 |pmid=2514360 |doi=}}</ref> Cocaine's rewarding effects come primarily from increased activity in the [[mesolimbic]] dopamine pathway.<ref name="pmid1719677">{{cite journal |author=Kuhar MJ, Ritz MC, Boja JW |title=The dopamine hypothesis of the reinforcing properties of cocaine |journal=Trends Neurosci. |volume=14 |issue=7 |pages=299–302 |year=1991 |pmid=1719677}}</ref> The three most common routes of administration for cocaine are intravenous, smoked and intranasal. All three routes appear to exert the same phamacological effect, and differ only in the speed of entrance into the brain.<ref>{{cite journal |author=Volkow ND, Wang GJ, Fischman MW, ''et al'' |title=Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain |journal=Life Sci. |volume=67 |issue=12 |pages=1507–15 |year=2000 |pmid=10983846 |doi=}}</ref> | |||
Cocaine has an anesthetic effect by inhibiting voltage-gated sodium (Na<sup>+</sup>) channels in nerve cells. These channels are essential in generating [[action potential]]s, and their blockage prevents nerves from transmitting information. Topical anesthetics have been developed from cocaine's structure to take advantage of this particular property. | |||
==Health Issues== | |||
Cocaine | ===Heart=== | ||
Cocaine can cause coronary artery spasms which lead to a myocardial infarction. This effect can happen randomly to any user. The coronary artery spasms can occur on the users first usage or any other usage after. The coronary spasms cause the ectopic ventricular foci of the heart to become hypoxic and the extreme irritability can trigger life-threatening ventricular arrhythmias. The risk of myocardial infartion increases 24 times the normal risk during the first hour after use of cocaine. | |||
==References== | ==References== | ||
<references/> | <references/> | ||
== External link == | |||
{{CZMed}}[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 06:00, 30 July 2024
Cocaine or benzoylmethyl ecgonine (IUPAC name methyl (2R,3S)-3-(benzoyloxy)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate) is an organic chemical compound that belongs to the alkaloid category. Its chemical formula is C17H21NO4. Cocaine is a colorless or white crystalline substance. It is primarily extracted from coca plant, largely grown in South America.
Cocaine is primarily used recreationally for its stimulating and euphoric effects on human body. Due to its abuse, the possession, production and distribution of cocaine has been declared illegal by most countries. In the United States, it is classified by the Controlled Substance Act as a Schedule II substance, allowing only limited medical use. Along with heroin and methamphetamine, cocaine is often considered a "hard drug", being much more physically addictive and harmful than a so-called "soft drug" like marijuana. Despite harsh penal codes against the consumption, production, and distribution of the drug, a black market is still flourishing. Street names for cocaine include coke, snow, and blow, among others.
History
Cocaine was first refined from raw coca in 1860 by Albert Niemann, a German graduate student working under Friedrich Wöhler in Göttingen University. In the decades after its original isolation, cocaine was used as an experimental drug in a wide variety of clinical applications by researchers including Sigmund Freud. [1] It soon came to be used in a variety of commercial products including some that were very popular, including Vin Mariani and Coca Cola. Cocaine production was seen as a significant economic resource and a modernizing export in Peru. It was not until after World War I that U.S. pressure abroad made cocaine an illicit product on the international stage.
Pharmacology
The majority of cocaine's effects are due to increasing monoamine levels by binding to serotonin (5-HT), norepinephrine (NE), and dopamine (DA) catecholamine plasma membrane transport proteins. This blockade prevents the reuptake of neurotransmitters, thus increasing the amount available at the synapse to bind to the post synaptic cell. In contrast to amphetamine, a similar psychomotor stimulant, cocaine is a dopamine uptake inhibitor and does not stimulate DA release.[2] Cocaine's rewarding effects come primarily from increased activity in the mesolimbic dopamine pathway.[3] The three most common routes of administration for cocaine are intravenous, smoked and intranasal. All three routes appear to exert the same phamacological effect, and differ only in the speed of entrance into the brain.[4]
Cocaine has an anesthetic effect by inhibiting voltage-gated sodium (Na+) channels in nerve cells. These channels are essential in generating action potentials, and their blockage prevents nerves from transmitting information. Topical anesthetics have been developed from cocaine's structure to take advantage of this particular property.
Health Issues
Heart
Cocaine can cause coronary artery spasms which lead to a myocardial infarction. This effect can happen randomly to any user. The coronary artery spasms can occur on the users first usage or any other usage after. The coronary spasms cause the ectopic ventricular foci of the heart to become hypoxic and the extreme irritability can trigger life-threatening ventricular arrhythmias. The risk of myocardial infartion increases 24 times the normal risk during the first hour after use of cocaine.
References
- ↑ Gootenberg, Paul (2009). Andean cocaine: the making of a global drug. Chapel Hill: University of North Carolina Press. ISBN 9780807859056.
- ↑ Gold LH, Geyer MA, Koob GF (1989). "Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs". NIDA Res. Monogr. 94: 101–26. PMID 2514360. [e]
- ↑ Kuhar MJ, Ritz MC, Boja JW (1991). "The dopamine hypothesis of the reinforcing properties of cocaine". Trends Neurosci. 14 (7): 299–302. PMID 1719677.
- ↑ Volkow ND, Wang GJ, Fischman MW, et al (2000). "Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain". Life Sci. 67 (12): 1507–15. PMID 10983846. [e]
External link
The most up-to-date information about Cocaine and other drugs can be found at the following sites.
- Cocaine - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Cocaine - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Cocaine - Detailed information from DrugBank.