Glycol dehydration: Difference between revisions
imported>Milton Beychok m (→Purpose: Fixed typo) |
mNo edit summary |
||
| (3 intermediate revisions by 2 users not shown) | |||
| Line 14: | Line 14: | ||
==Purpose== | ==Purpose== | ||
The purpose of a glycol dehydration unit is to remove water from natural gas and natural gas liquids. When produced from an underground reservoir, raw natural gas is usually saturated with water vapor at the water (i.e., the gas is at its water [[dew point]]). Without dehydration, liquid water could [[Condensation|condense]] out of the gas as it is either cooled or the [[pressure]] is lowered as the gas flows through [[Natural gas processing|processing facilities]] and [[pipeline]]s. This liquid water phase may contain some amount of [[acid gas]], such as [[hydrogen | The purpose of a glycol dehydration unit is to remove water from natural gas and natural gas liquids. When produced from an underground reservoir, raw natural gas is usually saturated with water vapor at the water (i.e., the gas is at its water [[dew point]]). Without dehydration, liquid water could [[Condensation (phase transition)|condense]] out of the gas as it is either cooled or the [[pressure]] is lowered as the gas flows through [[Natural gas processing|processing facilities]] and [[pipeline]]s. This liquid water phase may contain some amount of [[acid gas]], such as [[hydrogen sulphide]] (H<sub>2</sub>S) or [[carbon dioxide]] (CO<sub>2</sub>) and can cause [[corrosion]].<ref name=GPSA_Databook/> | ||
At low [[temperature]]s, [[methane hydrates]] (also known as methane [[clathrates]]) may form.<ref>Hydrates or clathrates are [[crystalline]] water-based solids physically resembling [[ice]], in which gas [[molecules]] are trapped inside "cages" of water molecules</ref> Depending on the natural gas composition, methane hydrates can form at relatively high temperatures and cause plugging of equipment and piping.<ref name=GPSA_Databook/> Drying the gas with glycol dehydration unit lowers the temperature at which hydrate formation will occur. | At low [[temperature]]s, [[methane hydrates]] (also known as methane [[clathrates]]) may form.<ref>Hydrates or clathrates are [[crystalline]] water-based solids physically resembling [[ice]], in which gas [[molecules]] are trapped inside "cages" of water molecules</ref> Depending on the natural gas composition, methane hydrates can form at relatively high temperatures and cause plugging of equipment and piping.<ref name=GPSA_Databook/> Drying the gas with glycol dehydration unit lowers the temperature at which hydrate formation will occur. | ||
| Line 35: | Line 35: | ||
'''''Wet gas knockout drum:''''' Some glycol dehydration facilities include routing of the wet gas feedstock through a [[vapor-liquid separator]] drum (also referred to as a ''knockout drum'') to remove any [[Entrainment (engineering)| entrained]] liquid water droplets before entering the glycol unit absorber. | '''''Wet gas knockout drum:''''' Some glycol dehydration facilities include routing of the wet gas feedstock through a [[vapor-liquid separator]] drum (also referred to as a ''knockout drum'') to remove any [[Entrainment (engineering)| entrained]] liquid water droplets before entering the glycol unit absorber. | ||
'''''Glycol filter:''''' Some glycol dehydration facilities include a [[filter]] within the circulating glycol system to remove any undesirable solid particles that could lead to plugging of the various equipment items. | '''''Glycol filter:''''' Some glycol dehydration facilities include a [[Filter (engineering)|filter]] within the circulating glycol system to remove any undesirable solid particles that could lead to plugging of the various equipment items. | ||
'''''Stripper top internal condenser:''''' Instead of the external condenser, reflux drum and reflux pump shown in the above schematic flow diagram, some glycol units use a coil (through which a coolant stream flows) installed directly in the top of the stripper. The exit water vapor stream is then vented directly from the top of the stripper. | '''''Stripper top internal condenser:''''' Instead of the water-cooled external [[condensation (phase transition)|condenser]], [[reflux]] drum and reflux pump shown in the above schematic flow diagram, some glycol units use a coil (through which a coolant stream flows) installed directly in the top of the stripper. The exit water vapor stream is then vented directly from the top of the stripper. Also, an air-cooled external condenser may be used instead of a water-cooled one. | ||
==Other configurations== | ==Other configurations== | ||
| Line 52: | Line 52: | ||
== References == | == References == | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:01, 22 August 2024
Glycol dehydration is a chemical engineering unit process that uses a liquid desiccant, usually a glycol, for the removal of water from natural gas and natural gas liquids (NGL). It is the most common and economic means of water removal from these streams.[1][2]
The glycols typically used in dehydration process units include:
- ethylene glycol (MEG), formula = C2H6O2
- diethylene glycol (DEG), formula = C4H10O3
- triethylene glycol (TEG), formula = C6H14O4
- tetraethylene glycol (TTEG), formula = C8H18O5
Of the four glycols listed above, triethylene glycol (TEG) is the one most commonly used in dehydrating natural gas.
Purpose
The purpose of a glycol dehydration unit is to remove water from natural gas and natural gas liquids. When produced from an underground reservoir, raw natural gas is usually saturated with water vapor at the water (i.e., the gas is at its water dew point). Without dehydration, liquid water could condense out of the gas as it is either cooled or the pressure is lowered as the gas flows through processing facilities and pipelines. This liquid water phase may contain some amount of acid gas, such as hydrogen sulphide (H2S) or carbon dioxide (CO2) and can cause corrosion.[1]
At low temperatures, methane hydrates (also known as methane clathrates) may form.[3] Depending on the natural gas composition, methane hydrates can form at relatively high temperatures and cause plugging of equipment and piping.[1] Drying the gas with glycol dehydration unit lowers the temperature at which hydrate formation will occur.
For the above reasons, pipeline quality specifications for natural gas usually mandate that the water content should not exceed 4 to 7 pounds per million SCF (68 to 118 mg per Nm3 ).[4] Glycol dehydration units must typically meet this specification at a minimum, although further removal may be required if additional hydrate formation temperature depression is required.
Process description
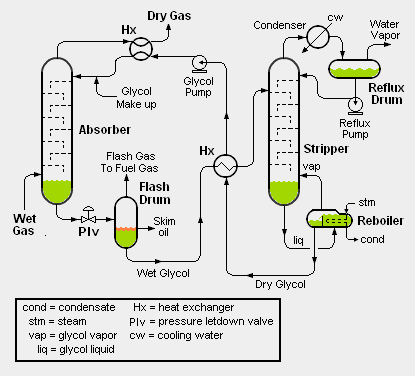
High pressure, wet natural gas is fed into the bottom of the absorber (see the adjacent schematic flow diagram) and flows upwards while contacting dry glycol flowing downwards. The glycol absorbs and removes water from the wet natural gas. The resulting dried natural gas leaves from the top of the absorber and cools the incoming hot, dry glycol in a heat exchanger before exiting the dehydration unit as dry natural gas.
After leaving the bottom of the absorber, the now wet glycol flows through a pressure control valve, referred to as the pressure letdown valve (see the adjacent flow diagram) and flows into the flash drum where it undergoes a flash evaporation to release any natural gas absorbed in the glycol. This step in the process is necessary as the pressure must be reduced before the water can be removed from the wet glycol in the stripper. Some of the hydrocarbon liquids that may be present in the natural gas will be entrained in the wet glycol from the absorber. Most of such hydrocarbon liquids will form a layer of oil on top of the wet liquid glycol and be removed as skim oil. The gas released by the flash evaporation is called flash gas and may be used as a fuel gas.
The wet glycol from the flash drum is heated (in a heat exchanger) by the hot dry glycol from the bottom of the stripper and then routed into to the stripper (also known as a regenerator)as shown in the adjacent diagram. The stripper removes the absorbed water from the wet glycol and produces a dry glycol of at least 99% purity (i.e., containing less than 1% water). After exchanging heat with the wet glycol from the flash drum, the dry glycol is pumped back for reuse as dry glycol entering the top of the absorber.
Both the absorber and the stripper may be trayed (as shown in the adjacent diagram) or packed columns. The stripper is, in essence, a simple distillation column and the distillation heat is supplied by a reboiler using steam or some other heat medium. The wet glycol and dry glycol are often referred to as rich glycol and lean glycol, respectively.
Other components
Wet gas knockout drum: Some glycol dehydration facilities include routing of the wet gas feedstock through a vapor-liquid separator drum (also referred to as a knockout drum) to remove any entrained liquid water droplets before entering the glycol unit absorber.
Glycol filter: Some glycol dehydration facilities include a filter within the circulating glycol system to remove any undesirable solid particles that could lead to plugging of the various equipment items.
Stripper top internal condenser: Instead of the water-cooled external condenser, reflux drum and reflux pump shown in the above schematic flow diagram, some glycol units use a coil (through which a coolant stream flows) installed directly in the top of the stripper. The exit water vapor stream is then vented directly from the top of the stripper. Also, an air-cooled external condenser may be used instead of a water-cooled one.
Other configurations
The process design of most glycol units are fairly uniform except for the regeneration step. Several methods are used to enhance the performance of the glycol stripper so as achieve lower levels of residual water vapor in the product dry glycol from the stripper. The lower is the water content of the dry glycol used in the absorber, the lower is the water content of the product dry gas from the absorber.
Since the reboiler temperature of the glycol stripper is limited to about 400 °F (about 200 °C) to prevent thermal degradation of the circulating glycol, many of the enhanced systems focus on lowering the partial pressure of water in the stripper to increase the removal of water from the wet glycol. Common enhancing methods include the use of stripping gas as well as reboil heat and lowering the total stripper pressure by using a vacuum system.
There are two proprietary process designs that are available:[2][5]
The DRIZO process design: The DRIZO design also focuses on improving the stripping of water from the glycol by using a recoverable hydrocarbon solvent instead of a stripping gas.
The Coldfinger process design: The main focus of this design is to condense and collect water and hydrocarbons from the vapor phase in the reboiler and drain it out of the stripper system. Any glycol also condensed is collected and periodically fed back into the stripper.
References
- ↑ 1.0 1.1 1.2 Gas Processors Suppliers Association (GPSA) Handbook, Tenth Edition.
- ↑ 2.0 2.1 Arthur L. Kohl and Fred C. Riesenfeld (1984). Gas Purification, 4th Edition. Gulf Publishing Co.. ISBN 0-87201-314-6.
- ↑ Hydrates or clathrates are crystalline water-based solids physically resembling ice, in which gas molecules are trapped inside "cages" of water molecules
- ↑ SCF = standard cubic feet of gas at 60 °F and 1 atm. Nm3 = normal cubic meters of gas at 0 °C and 1 atm. 1 Nm3 = 37.326 SCF.
- ↑ Glycol Dehydration