Zidovudine: Difference between revisions
imported>David E. Volk |
mNo edit summary |
||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 17: | Line 17: | ||
}} | }} | ||
'''Zidovudine''' is an [[antiviral drug]] used primarily for the treatment of [[ | '''Zidovudine''' is an [[antiviral drug]] used primarily for the treatment of [[Human Immunodeficiency Virus Type 1]] (HIV-1). | ||
== Mechanism of action == | == Mechanism of action == | ||
Zidovudine is a nucleoside-based [[reverse transcriptase]] [[reverse transcriptase inhibitor|inhibitor]] that is structurally analogous to [[thymidine]], a normal base in DNA. After phosphorylation, it becomes incorporated into viral DNA where it acts as a DNA chain terminator. The viral DNA chain is terminated because the 3'-hydroxy group needed for the formation of a | Zidovudine is a nucleoside-based [[reverse transcriptase]] [[reverse transcriptase inhibitor|inhibitor]] that is structurally analogous to [[thymidine]], a normal base in DNA. After phosphorylation, it becomes incorporated into viral DNA where it acts as a DNA chain terminator. The viral DNA chain is terminated because the 3'-hydroxy group needed for the formation of a phosphodiester bond is missing, having been replaced by an azido group. It also inhibits the HIV RT by binding with it and thus competing with natural substrates. | ||
== Chemistry == | == Chemistry == | ||
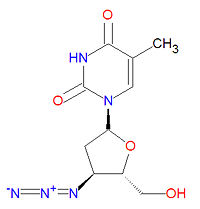
The IUPAC chemical name for zidovudine is 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione and it has chemical formula C<sub>10</sub>H<sub>13</sub>N<sub>5</sub>O<sub>4</sub>, giving it a molecular mass of 267.2413 g/mol. It is dideoxynucleoside in which the usual 3'-hydroxy group has been replaced by an azido group, making incapable of forming normal phosphodiester bonds required for normal DNA. | The IUPAC chemical name for zidovudine is 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione and it has chemical formula C<sub>10</sub>H<sub>13</sub>N<sub>5</sub>O<sub>4</sub>, giving it a molecular mass of 267.2413 g/mol. It is dideoxynucleoside in which the usual 3'-hydroxy group has been replaced by an azido group, making incapable of forming normal phosphodiester bonds required for normal DNA. | ||
==History== | |||
In the United States, it was approved with a [http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/NewDrugApplicationNDA/ New Drug Application] (NDA) by the FDA in 1987.<ref>[http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.SearchAction&SearchType=BasicSearch&Search_Button=Submit&searchTerm=019655 Drugs@FDA]. U S Food and Drug Administration</ref> A generic version was approved with a [http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ Abbreviated New Drug Application] (ANDA) in 1995.<ref>[http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.SearchAction&SearchType=BasicSearch&Search_Button=Submit&searchTerm=074047 Drugs@FDA]. U S Food and Drug Administration</ref> | |||
==Clinical uses== | |||
AZT can benefit patients with [[Human Immunodeficiency Virus ]].<ref name="pmid3299089">{{cite journal |author=Fischl MA, Richman DD, Grieco MH, ''et al.'' |title=The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial |journal=N. Engl. J. Med. |volume=317 |issue=4 |pages=185–91 |year=1987 |month=July |pmid=3299089 |doi= |url= |issn=}}</ref> | |||
==References== | |||
<references/> | |||
[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 12:00, 10 November 2024
|
| |||||||
| zidovudine | |||||||
| |||||||
| Uses: | antiviral drug HIV | ||||||
| Properties: | reverse transcriptase inhibitor | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Zidovudine is an antiviral drug used primarily for the treatment of Human Immunodeficiency Virus Type 1 (HIV-1).
Mechanism of action
Zidovudine is a nucleoside-based reverse transcriptase inhibitor that is structurally analogous to thymidine, a normal base in DNA. After phosphorylation, it becomes incorporated into viral DNA where it acts as a DNA chain terminator. The viral DNA chain is terminated because the 3'-hydroxy group needed for the formation of a phosphodiester bond is missing, having been replaced by an azido group. It also inhibits the HIV RT by binding with it and thus competing with natural substrates.
Chemistry
The IUPAC chemical name for zidovudine is 1-[(2R,4S,5S)-4-azido-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione and it has chemical formula C10H13N5O4, giving it a molecular mass of 267.2413 g/mol. It is dideoxynucleoside in which the usual 3'-hydroxy group has been replaced by an azido group, making incapable of forming normal phosphodiester bonds required for normal DNA.
History
In the United States, it was approved with a New Drug Application (NDA) by the FDA in 1987.[1] A generic version was approved with a Abbreviated New Drug Application (ANDA) in 1995.[2]
Clinical uses
AZT can benefit patients with Human Immunodeficiency Virus .[3]
References
- ↑ Drugs@FDA. U S Food and Drug Administration
- ↑ Drugs@FDA. U S Food and Drug Administration
- ↑ Fischl MA, Richman DD, Grieco MH, et al. (July 1987). "The efficacy of azidothymidine (AZT) in the treatment of patients with AIDS and AIDS-related complex. A double-blind, placebo-controlled trial". N. Engl. J. Med. 317 (4): 185–91. PMID 3299089. [e]