Polymer: Difference between revisions
imported>Jacob Jensen (Significant copyediting and reworking but still not ready to go live) |
mNo edit summary |
||
| (13 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
A '''polymer''' is a substance composed of molecules with large [[molecular mass]] consisting of repeating [[structural unit]]s, or [[monomer]]s, connected by [[covalent]] [[chemical bond]]s. The individual molecules which comprise a polymer are referred to as '''polymer molecules''', where the word "polymer" functions as an adjective.<ref>IUPAC. "Glossary of Basic Terms in Polymer Science". Pure Appl. Chem. 1996, 68, 2287-2311.</ref> | {{subpages}} | ||
A '''polymer''' is a substance composed of molecules with large [[molecular mass]] consisting of repeating [[structural unit]]s, or [[monomer]]s, connected by [[covalent]] [[chemical bond]]s. The individual molecules which comprise a polymer are referred to as '''polymer molecules''', where the word "polymer" functions as an adjective.<ref>IUPAC. "Glossary of Basic Terms in Polymer Science". Pure Appl. Chem. 1996, 68, 2287-2311.</ref> | |||
{{Image|Polystyrene formation.png|right|500px|Formation of the '''polymer''' [[polystyrene]] from [[styrene]] monomers.<br />The squiggly lines mean that only part of the polymer chain is shown.}} | |||
==Overview== | ==Overview== | ||
| Line 5: | Line 8: | ||
While the term polymer in popular usage suggests "[[plastic]]", polymers comprise a large class of natural and synthetic materials with a variety of properties and purposes. Natural polymer materials such as [[shellac]] and [[amber]] have been in use for centuries. Paper is manufactured from [[cellulose]], a naturally occurring [[polysaccharide]] found in plants. Biopolymers such as [[proteins]] and [[nucleic acids]] play important roles in biological processes. | While the term polymer in popular usage suggests "[[plastic]]", polymers comprise a large class of natural and synthetic materials with a variety of properties and purposes. Natural polymer materials such as [[shellac]] and [[amber]] have been in use for centuries. Paper is manufactured from [[cellulose]], a naturally occurring [[polysaccharide]] found in plants. Biopolymers such as [[proteins]] and [[nucleic acids]] play important roles in biological processes. | ||
[[Henri Braconnot]]'s pioneering work in derivative cellulose compounds is perhaps the earliest important work in modern polymer science. The development of [[vulcanization]] later in the nineteenth century improved the durability of the natural polymer [[rubber]], signifying the first popularized semi-synthetic polymer. The first wholly synthetic polymer, [[Bakelite]], was | [[Henri Braconnot]]'s pioneering work in derivative cellulose compounds is perhaps the earliest important work in modern polymer science. The development of [[vulcanization]] later in the nineteenth century improved the durability of the natural polymer [[rubber]], signifying the first popularized semi-synthetic polymer. The first wholly synthetic polymer, [[Bakelite]], was discovered in 1907. | ||
Until the 1920s, most scientists believed that polymers were clusters of small molecules (called colloids), without definite molecular weights, held together by an unknown force, a concept known as [[association theory]]. In 1922, German chemist [[Hermann Staudinger]] proposed that polymers were comprised of "[[macromolecules]]" consisted of long chains of atoms held together by covalent bonds. Though poorly received at first, experimental work by [[Wallace Carothers]], [[Herman Mark]], and others provided further evidence for Staudinger's theory. By the mid-1930s, the macromolecular theory of polymer structure was widely accepted. For this and other work in the field, Staudinger was ultimately awarded the [[Nobel Prize]]. In the intervening century, synthetic polymer materials such as [[Nylon]], [[polyethylene]], [[Teflon]], and [[silicone]] have formed the basis for a burgeoning polymer industry. | Until the 1920s, most scientists believed that polymers were clusters of small molecules (called colloids), without definite molecular weights, held together by an unknown force, a concept known as [[association theory]]. In 1922, German chemist [[Hermann Staudinger]] proposed that polymers were comprised of "[[macromolecules]]" consisted of long chains of atoms held together by covalent bonds. Though poorly received at first, experimental work by [[Wallace Carothers]], [[Herman Mark]], and others provided further evidence for Staudinger's theory. By the mid-1930s, the macromolecular theory of polymer structure was widely accepted. For this and other work in the field, Staudinger was ultimately awarded the [[Nobel Prize]]. In the intervening century, synthetic polymer materials such as [[Nylon]], [[polyethylene]], [[Teflon]], and [[silicone]] have formed the basis for a burgeoning polymer industry. | ||
| Line 15: | Line 18: | ||
''See Also: [[Polymer science]]'' | ''See Also: [[Polymer science]]'' | ||
Most polymer research may be categorized as [[polymer science]], a sub-discipline of [[materials science]] which includes researchers in [[chemistry]] (especially [[organic chemistry]]), [[physics]], and [[engineering]]. The field of polymer science includes both experimental and theoretical research. The IUPAC recommends that polymer science be roughly divided into two subdisciplines: | Most polymer research may be categorized as [[polymer science]], a sub-discipline of [[materials science]] which includes researchers in [[chemistry]] (especially [[organic chemistry]]), [[physics]], and [[engineering]]. The field of polymer science includes both experimental and theoretical research. The IUPAC recommends that polymer science be roughly divided into two subdisciplines: [[polymer chemistry]] (or [[macromolecular chemistry]]) and [[polymer physics]]. In practice the distinction between the two is rarely clearcut. | ||
The study of biological polymers, their structure, function, and method of synthesis is generally the purview of [[biology]], [[biochemistry]], and [[biophysics]]. These disciplines share some of the terminology familiar to polymer science, especially when describing the synthesis of biopolymers such as DNA or polysaccharides. However, usage differences persist, such as the practice of using the term [[macromolecule]] to describe large non-polymer molecules and complexes of multiple molecular components, such as [[hemoglobin]]. Substances with distinct biological function are rarely described in the terminology of polymer science. For example, a [[protein]] is rarely referred to as a [[copolymer]]. | The study of biological polymers, their structure, function, and method of synthesis is generally the purview of [[biology]], [[biochemistry]], and [[biophysics]]. These disciplines share some of the terminology familiar to polymer science, especially when describing the synthesis of biopolymers such as DNA or polysaccharides. However, usage differences persist, such as the practice of using the term [[macromolecule]] to describe large non-polymer molecules and complexes of multiple molecular components, such as [[hemoglobin]]. Substances with distinct biological function are rarely described in the terminology of polymer science. For example, a [[protein]] is rarely referred to as a [[copolymer]]. | ||
| Line 23: | Line 24: | ||
==Polymer synthesis== | ==Polymer synthesis== | ||
Polymers are synthesized by three primary methods: organic synthesis in a laboratory or factory, biological synthesis in living cells | Polymers are created, or synthesized, by three primary methods: biological synthesis in living cells and organisms, chemical modification of naturally occurring polymers, and pure organic synthesis in a laboratory or factory. Of the three, biological synthesis is perhaps the most complex, enabling the formation of large macromolecules with structure and function custom-tailored to particular biological applications. | ||
Natural polymers and biopolymers formed in living cells are synthesized by enzyme-mediated processes, such as the formation of [[DNA]] catalyzed by [[DNA polymerase]]. The [[Protein biosynthesis|synthesis of proteins]] involves multiple enzyme-mediated processes to [[Transcription (genetics)|transcribe]] genetic information from the DNA and subsequently [[Translation (biology)|translate]] that information to synthesize the specified protein. The protein may be [[Posttranslational modification|modified further]] following translation in order to provide appropriate structure and function. | |||
Early work in modern polymer science involved the chemical modification of simple, naturally occurring polymers. Prominent early examples include the reaction of nitric acid and cellulose to form [[nitrocellulose]] and the vulcanization process employed to increase the durability of rubber. | |||
The earliest example of a laboratory-synthesized polymer is [[Bakelite]], a hard resin discovered by [[Leo Baekeland]] in 1907 formed by the reaction of phenol and formaldehyde.. [[Bakelite]], was produced by reacting phenol and formaldehyde at precisely controlled temperature and pressure. Subsequent work by [[Wallace Carothers]] in the 1920s demonstrated that polymers could be synthesized rationally from their constituent monomers. The intervening years have shown significant developments in rational polymer synthesis. Most commercially important polymers today are entirely synthetic, produced in high volume on appropriately scaled organic synthetic techniques. | |||
Laboratory synthetic methods are generally divided into two categories, [[chain-growth polymerization]] and [[addition polymerization]] though some newer methods, such as [[plasma polymerization]] do not neatly fit into either category. Synthetic polymerization reactions may be carried out with or without a catalyst. Efforts towards rational synthesis of biopolymers via laboratory synthetic methods, especially artificial [[peptide synthesis|synthesis of proteins]], is an area of intense research. | Laboratory synthetic methods are generally divided into two categories, [[chain-growth polymerization]] and [[addition polymerization]] though some newer methods, such as [[plasma polymerization]] do not neatly fit into either category. Synthetic polymerization reactions may be carried out with or without a catalyst. Efforts towards rational synthesis of biopolymers via laboratory synthetic methods, especially artificial [[peptide synthesis|synthesis of proteins]], is an area of intense research. | ||
=== | ==Describing polymers== | ||
===Molecular description=== | |||
In many instances a polymer molecule may be described using the same terminology used to describe any molecule. Molecular structure may be described in terms of bond lengths and angles; mass may be described in terms of molecular weight. However, the size and complexitiy of polymer molecules often require the use of specialized terminology to precisely describe the nature of the monomer(s) and how those monomers are connected or arranged. | |||
The mass of a polymer molecule may be described using standard conventional for molecular or molar mass. This molecular weight, expressed in Daltons, is used frequently to describe complex biopolymers, such as proteins. An altnerate expression for molecular mass is the [[degree of polymerization]] (DP), essentially the number of monomer units which comprise the polymer or block. DP is especially convenient for homopolymers or block copolymers, and is used frequency when describing the extent of a polymerization reaction. | |||
Laboratory syntheses tend to produce polymer samples comprised of molecules with a variety of different molecular weights. Thus, the molecular weight of a synthetic polymer is generally expressed in terms of a summary statistic, such as the number-averaged molecular weight (M<sub>n</sub>) or weight-averaged molecular weight(M<sub>w</sub>). The ratio of these two values is known as the [[polydispersity index]], which is an indicator of the distribution of molecular weights within a polymer sample. For commercial polymer samples it is common to see all three values reported. | |||
The size and complexity of polymer molecules also requires specialized descriptions of molecular dimensions and ordering. Globular macromolecules, such as proteins, may have a well-defined semi-rigid structure where conventional descriptions of atomic positions, bond lengths, and angles are adequate and appropriate. On the other hand, structurally simple polymers, such as linear polymers, possess hundreds or thousands of degrees of rotational freedom, allowing the polymer chain to adopt multiple conformations. The size and positions of such molecules are described statistically, with molecular volume expressed as a function of the [[radius of gyration]] or mean end-to-end distance. Molecular simulations or light scattering may also be used to determine an energetically favorable "average conformation" for a collection of polymer molecules. | |||
Describing the crystallinity of a polymer presents some degree of ambiguity. In some cases, the term crystalline finds identical usage to that used in conventional [[crystallography]]. For example, the structure of a crystalline protein or polynucleotide, such as a sample prepared for [[x-ray crystallography]], may be defined in terms of a conventional unit cell comprised of one or more polymer molecules with cell dimensions of hundreds of angstroms or more. | |||
When applied to a synthetic polymer, however, the term crystalline implies regions of three-dimensional ordering on atomic (rather than macromolecular) length scales, usually arising from intramolecular folding and/or stacking of adjacent chains. Synthetic polymers may consist of both crystalline and amorphous regions; the '''degree of crystallinity''' may be expressed in terms of a weight fraction or volume fraction of crystalline material. Few synthetic polymers are entirely crystalline.<ref>http://www.iupac.org/publications/books/pbook/PurpleBook-C4.pdf</ref> | |||
A stereochemical description of a polymer molecule also requires specialized terminology. There are some polymers in which chirality in the monomer is retained following polymerization. In other polymers a chiral center may be created by the polymerization process. | |||
There is also a large vocabulary used to describe the nature of the monomers and the precise arrangement of these monomers relative to one another. A polymer consisting of exactly one type of monomer, such as poly(styrene), is classifed as a '''homopolymer'''. Polymers with more than one variety of monomer are called [[copolymers]], such as [[ethylene-vinyl acetate]]. Some biological polymers are composed of a variety of different but structurally related monomers, such as [[polynucleotides]] composed of [[nucleotide]] subunits. | |||
A '''[[polyelectrolyte]]''' molecule is a polymer molecule comprised of primarily ionizable repeating subunits. An '''ionomer''' molecule is also ionizable, but to a lesser degree. | A '''[[polyelectrolyte]]''' molecule is a polymer molecule comprised of primarily ionizable repeating subunits. An '''ionomer''' molecule is also ionizable, but to a lesser degree. | ||
The simplest form of polymer molecule is a straight chain or '''linear''' polymer, composed of a single main chain. A '''[[branching (chemistry)|branched polymer]]''' molecule is composed of a main chain with one or more substituent side chains or branches. Special types of branched polymers include star polymers, comb polymers, and brush polymers. If the polymer contains a side chain that has a different composition or configuration than the main chain the polymer is called a [[graft copolymer|graft or grafted polymer]]. A '''cross-link''' suggests a branch point from which four or more distinct chains emanate. A polymer molecule with a high degree of crosslinking is referred to as a '''polymer network'''.<ref>IUPAC. "Glossary of Basic Terms in Polymer Science". Pure Appl. Chem. 1996, 68, 2287-2311.</ref> | The simplest form of polymer molecule is a straight chain or '''linear''' polymer, composed of a single main chain. A '''[[branching (chemistry)|branched polymer]]''' molecule is composed of a main chain with one or more substituent side chains or branches. Special types of branched polymers include star polymers, comb polymers, and brush polymers. If the polymer contains a side chain that has a different composition or configuration than the main chain the polymer is called a [[graft copolymer|graft or grafted polymer]]. A '''cross-link''' suggests a branch point from which four or more distinct chains emanate. A polymer molecule with a high degree of crosslinking is referred to as a '''polymer network'''.<ref>IUPAC. "Glossary of Basic Terms in Polymer Science". Pure Appl. Chem. 1996, 68, 2287-2311.</ref> | ||
===Macroscopic description=== | |||
== | |||
The properties of polymers | The macroscopic physical properties of polymers in many cases reflect that of any other molecular substance. Polymer materials may be transparent, translucent, or opaque, and may be insulators, conductors, or semiconductors. Other properties, however, especially those governing phase transitions, may have distinct meanings (or no meaning at all) when applied to polymers. | ||
The "melting point" of a polymer, for example, refers not a solid-liquid phase transition but a transition from a crystalline or semi-crystalline phase to a solid amorphous phase. Though abbreviated as simply "T<sub>m</sub>", the property in question is more properly called the "crystalline melting temperature". Among synthetic polymers, crystalline melting is only discussed with regards to [[thermoplastics]], as [[thermoset|thermosetting]] polymers will decompose at high temperatures rather than melt. | |||
The boiling point of a polymer substance is never defined, in that polymers will decompose before reaching assumed boiling temperatures. | The boiling point of a polymer substance is never defined, in that polymers will decompose before reaching assumed boiling temperatures. | ||
A parameter of particular interest in synthetic polymer manufacturing is the [[glass transition temperature]] (T<sub>g</sub>), which describes the temperature at which amorphous polymers undergo a second order phase transition from a rubbery, viscous amorphous solid to a brittle, glassy amorphous solid. The glass transition temperature may be engineered by altering the degree of branching or cross-linking in the polymer or by the addition of [[plasticizer]].<ref> Brandrup, J.; Immergut, E.H.; Grulke, E.A.; ''eds'' Polymer Handbook 4th Ed. New York: Wiley-Interscience, 1999. </ref> | A parameter of particular interest in synthetic polymer manufacturing is the [[glass transition temperature]] (T<sub>g</sub>), which describes the temperature at which amorphous polymers undergo a second order phase transition from a rubbery, viscous amorphous solid to a brittle, glassy amorphous solid. The glass transition temperature may be engineered by altering the degree of branching or cross-linking in the polymer or by the addition of [[plasticizer]].<ref> Brandrup, J.; Immergut, E.H.; Grulke, E.A.; ''eds'' Polymer Handbook 4th Ed. New York: Wiley-Interscience, 1999. </ref> | ||
==Standardized polymer nomenclature== | ==Standardized polymer nomenclature== | ||
There are multiple conventions for naming polymer substances. Many commonly used polymers, such as those found in consumer products, are referred to by a ''common'' or ''trivial'' name. The trivial name is assigned based on historical precedent or popular usage rather than a standardized naming convention. Both the [[American Chemical Society]]<ref>CAS: Index Guide, Appendix IV (© 1998). </ref> and [[IUPAC]]<ref>IUPAC. "Nomenclature of Regular Single-Strand Organic Polymers". Pure Appl. Chem. 1976, 48, 373-385. </ref> have proposed standardized naming conventions; the [[ACS]] and [[IUPAC]] conventions are similar but not identical. | There are multiple conventions for naming polymer substances. Many commonly used polymers, such as those found in consumer products, are referred to by a ''common'' or ''trivial'' name. The trivial name is assigned based on historical precedent or popular usage rather than a standardized naming convention. Both the [[American Chemical Society]]<ref>CAS: Index Guide, Appendix IV (© 1998). </ref> and [[IUPAC]]<ref>IUPAC. "Nomenclature of Regular Single-Strand Organic Polymers". Pure Appl. Chem. 1976, 48, 373-385. </ref> have proposed standardized naming conventions; the [[ACS]] and [[IUPAC]] conventions are similar but not identical. Examples of the difference between the various naming conventions are given in the table below: | ||
{| class="wikitable" | {| class="wikitable" | ||
| Line 92: | Line 79: | ||
|- | |- | ||
| Poly(ethylene oxide) or (PEO) | | [[Poly(ethylene oxide)]] or (PEO) | ||
| poly(oxyethylene) | | poly(oxyethylene) | ||
| poly(oxyethylene) | | poly(oxyethylene) | ||
|- | |- | ||
| Poly(ethylene terephthalate) or (PET) | | [[Poly(ethylene terephthalate)]] or (PET) | ||
| poly(oxy-1,2-ethanediyloxycarbonyl -1,4-phenylenecarbonyl) | | poly(oxy-1,2-ethanediyloxycarbonyl -1,4-phenylenecarbonyl) | ||
| poly(oxyethyleneoxyterephth= aloyl) | | poly(oxyethyleneoxyterephth= aloyl) | ||
|- | |- | ||
| Nylon | | [[Nylon 6]] | ||
| poly[imino(1-oxo-1,6-hexanediyl)] | | poly[imino(1-oxo-1,6-hexanediyl)] | ||
| poly[imino(1-oxohexane-1,6-diyl)] | | poly[imino(1-oxohexane-1,6-diyl)] | ||
| Line 112: | Line 99: | ||
==References== | ==References== | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 16:00, 5 October 2024
A polymer is a substance composed of molecules with large molecular mass consisting of repeating structural units, or monomers, connected by covalent chemical bonds. The individual molecules which comprise a polymer are referred to as polymer molecules, where the word "polymer" functions as an adjective.[1]
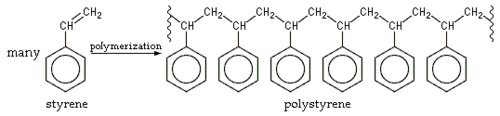
The squiggly lines mean that only part of the polymer chain is shown.
Overview
While the term polymer in popular usage suggests "plastic", polymers comprise a large class of natural and synthetic materials with a variety of properties and purposes. Natural polymer materials such as shellac and amber have been in use for centuries. Paper is manufactured from cellulose, a naturally occurring polysaccharide found in plants. Biopolymers such as proteins and nucleic acids play important roles in biological processes.
Henri Braconnot's pioneering work in derivative cellulose compounds is perhaps the earliest important work in modern polymer science. The development of vulcanization later in the nineteenth century improved the durability of the natural polymer rubber, signifying the first popularized semi-synthetic polymer. The first wholly synthetic polymer, Bakelite, was discovered in 1907.
Until the 1920s, most scientists believed that polymers were clusters of small molecules (called colloids), without definite molecular weights, held together by an unknown force, a concept known as association theory. In 1922, German chemist Hermann Staudinger proposed that polymers were comprised of "macromolecules" consisted of long chains of atoms held together by covalent bonds. Though poorly received at first, experimental work by Wallace Carothers, Herman Mark, and others provided further evidence for Staudinger's theory. By the mid-1930s, the macromolecular theory of polymer structure was widely accepted. For this and other work in the field, Staudinger was ultimately awarded the Nobel Prize. In the intervening century, synthetic polymer materials such as Nylon, polyethylene, Teflon, and silicone have formed the basis for a burgeoning polymer industry.
Synthetic polymers today find application in nearly every industry and area of life. Polymers are used in the fabrication of microprocessors, the development of new pharmaceuticals, and improving yield in petroleum extraction. Polymers are used as adhesives and lubricants, as well as structural components for products ranging from childrens' toys to aircraft. Future applications include polymeric transistors and substrates for flexible components and displays, enhanced drug delivery methods, and the development of smart materials.
Polymer science
See Also: Polymer science
Most polymer research may be categorized as polymer science, a sub-discipline of materials science which includes researchers in chemistry (especially organic chemistry), physics, and engineering. The field of polymer science includes both experimental and theoretical research. The IUPAC recommends that polymer science be roughly divided into two subdisciplines: polymer chemistry (or macromolecular chemistry) and polymer physics. In practice the distinction between the two is rarely clearcut.
The study of biological polymers, their structure, function, and method of synthesis is generally the purview of biology, biochemistry, and biophysics. These disciplines share some of the terminology familiar to polymer science, especially when describing the synthesis of biopolymers such as DNA or polysaccharides. However, usage differences persist, such as the practice of using the term macromolecule to describe large non-polymer molecules and complexes of multiple molecular components, such as hemoglobin. Substances with distinct biological function are rarely described in the terminology of polymer science. For example, a protein is rarely referred to as a copolymer.
Polymer synthesis
Polymers are created, or synthesized, by three primary methods: biological synthesis in living cells and organisms, chemical modification of naturally occurring polymers, and pure organic synthesis in a laboratory or factory. Of the three, biological synthesis is perhaps the most complex, enabling the formation of large macromolecules with structure and function custom-tailored to particular biological applications.
Natural polymers and biopolymers formed in living cells are synthesized by enzyme-mediated processes, such as the formation of DNA catalyzed by DNA polymerase. The synthesis of proteins involves multiple enzyme-mediated processes to transcribe genetic information from the DNA and subsequently translate that information to synthesize the specified protein. The protein may be modified further following translation in order to provide appropriate structure and function.
Early work in modern polymer science involved the chemical modification of simple, naturally occurring polymers. Prominent early examples include the reaction of nitric acid and cellulose to form nitrocellulose and the vulcanization process employed to increase the durability of rubber.
The earliest example of a laboratory-synthesized polymer is Bakelite, a hard resin discovered by Leo Baekeland in 1907 formed by the reaction of phenol and formaldehyde.. Bakelite, was produced by reacting phenol and formaldehyde at precisely controlled temperature and pressure. Subsequent work by Wallace Carothers in the 1920s demonstrated that polymers could be synthesized rationally from their constituent monomers. The intervening years have shown significant developments in rational polymer synthesis. Most commercially important polymers today are entirely synthetic, produced in high volume on appropriately scaled organic synthetic techniques.
Laboratory synthetic methods are generally divided into two categories, chain-growth polymerization and addition polymerization though some newer methods, such as plasma polymerization do not neatly fit into either category. Synthetic polymerization reactions may be carried out with or without a catalyst. Efforts towards rational synthesis of biopolymers via laboratory synthetic methods, especially artificial synthesis of proteins, is an area of intense research.
Describing polymers
Molecular description
In many instances a polymer molecule may be described using the same terminology used to describe any molecule. Molecular structure may be described in terms of bond lengths and angles; mass may be described in terms of molecular weight. However, the size and complexitiy of polymer molecules often require the use of specialized terminology to precisely describe the nature of the monomer(s) and how those monomers are connected or arranged.
The mass of a polymer molecule may be described using standard conventional for molecular or molar mass. This molecular weight, expressed in Daltons, is used frequently to describe complex biopolymers, such as proteins. An altnerate expression for molecular mass is the degree of polymerization (DP), essentially the number of monomer units which comprise the polymer or block. DP is especially convenient for homopolymers or block copolymers, and is used frequency when describing the extent of a polymerization reaction.
Laboratory syntheses tend to produce polymer samples comprised of molecules with a variety of different molecular weights. Thus, the molecular weight of a synthetic polymer is generally expressed in terms of a summary statistic, such as the number-averaged molecular weight (Mn) or weight-averaged molecular weight(Mw). The ratio of these two values is known as the polydispersity index, which is an indicator of the distribution of molecular weights within a polymer sample. For commercial polymer samples it is common to see all three values reported.
The size and complexity of polymer molecules also requires specialized descriptions of molecular dimensions and ordering. Globular macromolecules, such as proteins, may have a well-defined semi-rigid structure where conventional descriptions of atomic positions, bond lengths, and angles are adequate and appropriate. On the other hand, structurally simple polymers, such as linear polymers, possess hundreds or thousands of degrees of rotational freedom, allowing the polymer chain to adopt multiple conformations. The size and positions of such molecules are described statistically, with molecular volume expressed as a function of the radius of gyration or mean end-to-end distance. Molecular simulations or light scattering may also be used to determine an energetically favorable "average conformation" for a collection of polymer molecules.
Describing the crystallinity of a polymer presents some degree of ambiguity. In some cases, the term crystalline finds identical usage to that used in conventional crystallography. For example, the structure of a crystalline protein or polynucleotide, such as a sample prepared for x-ray crystallography, may be defined in terms of a conventional unit cell comprised of one or more polymer molecules with cell dimensions of hundreds of angstroms or more.
When applied to a synthetic polymer, however, the term crystalline implies regions of three-dimensional ordering on atomic (rather than macromolecular) length scales, usually arising from intramolecular folding and/or stacking of adjacent chains. Synthetic polymers may consist of both crystalline and amorphous regions; the degree of crystallinity may be expressed in terms of a weight fraction or volume fraction of crystalline material. Few synthetic polymers are entirely crystalline.[2]
A stereochemical description of a polymer molecule also requires specialized terminology. There are some polymers in which chirality in the monomer is retained following polymerization. In other polymers a chiral center may be created by the polymerization process.
There is also a large vocabulary used to describe the nature of the monomers and the precise arrangement of these monomers relative to one another. A polymer consisting of exactly one type of monomer, such as poly(styrene), is classifed as a homopolymer. Polymers with more than one variety of monomer are called copolymers, such as ethylene-vinyl acetate. Some biological polymers are composed of a variety of different but structurally related monomers, such as polynucleotides composed of nucleotide subunits.
A polyelectrolyte molecule is a polymer molecule comprised of primarily ionizable repeating subunits. An ionomer molecule is also ionizable, but to a lesser degree.
The simplest form of polymer molecule is a straight chain or linear polymer, composed of a single main chain. A branched polymer molecule is composed of a main chain with one or more substituent side chains or branches. Special types of branched polymers include star polymers, comb polymers, and brush polymers. If the polymer contains a side chain that has a different composition or configuration than the main chain the polymer is called a graft or grafted polymer. A cross-link suggests a branch point from which four or more distinct chains emanate. A polymer molecule with a high degree of crosslinking is referred to as a polymer network.[3]
Macroscopic description
The macroscopic physical properties of polymers in many cases reflect that of any other molecular substance. Polymer materials may be transparent, translucent, or opaque, and may be insulators, conductors, or semiconductors. Other properties, however, especially those governing phase transitions, may have distinct meanings (or no meaning at all) when applied to polymers.
The "melting point" of a polymer, for example, refers not a solid-liquid phase transition but a transition from a crystalline or semi-crystalline phase to a solid amorphous phase. Though abbreviated as simply "Tm", the property in question is more properly called the "crystalline melting temperature". Among synthetic polymers, crystalline melting is only discussed with regards to thermoplastics, as thermosetting polymers will decompose at high temperatures rather than melt.
The boiling point of a polymer substance is never defined, in that polymers will decompose before reaching assumed boiling temperatures.
A parameter of particular interest in synthetic polymer manufacturing is the glass transition temperature (Tg), which describes the temperature at which amorphous polymers undergo a second order phase transition from a rubbery, viscous amorphous solid to a brittle, glassy amorphous solid. The glass transition temperature may be engineered by altering the degree of branching or cross-linking in the polymer or by the addition of plasticizer.[4]
Standardized polymer nomenclature
There are multiple conventions for naming polymer substances. Many commonly used polymers, such as those found in consumer products, are referred to by a common or trivial name. The trivial name is assigned based on historical precedent or popular usage rather than a standardized naming convention. Both the American Chemical Society[5] and IUPAC[6] have proposed standardized naming conventions; the ACS and IUPAC conventions are similar but not identical. Examples of the difference between the various naming conventions are given in the table below:
| Common Name | ACS Name | IUPAC Name |
|---|---|---|
| Poly(ethylene oxide) or (PEO) | poly(oxyethylene) | poly(oxyethylene) |
| Poly(ethylene terephthalate) or (PET) | poly(oxy-1,2-ethanediyloxycarbonyl -1,4-phenylenecarbonyl) | poly(oxyethyleneoxyterephth= aloyl) |
| Nylon 6 | poly[imino(1-oxo-1,6-hexanediyl)] | poly[imino(1-oxohexane-1,6-diyl)] |
In both standardized conventions the polymers names are intended to reflect the monomer(s) from which they are synthesized rather than the precise nature of the repeating subunit. For example, the polymer synthesized from the simple alkene ethene is called polyethylene, retaining the -ene suffix even though the double bond is removed during the polymerization process.
References
- ↑ IUPAC. "Glossary of Basic Terms in Polymer Science". Pure Appl. Chem. 1996, 68, 2287-2311.
- ↑ http://www.iupac.org/publications/books/pbook/PurpleBook-C4.pdf
- ↑ IUPAC. "Glossary of Basic Terms in Polymer Science". Pure Appl. Chem. 1996, 68, 2287-2311.
- ↑ Brandrup, J.; Immergut, E.H.; Grulke, E.A.; eds Polymer Handbook 4th Ed. New York: Wiley-Interscience, 1999.
- ↑ CAS: Index Guide, Appendix IV (© 1998).
- ↑ IUPAC. "Nomenclature of Regular Single-Strand Organic Polymers". Pure Appl. Chem. 1976, 48, 373-385.