Sulfur dioxide: Difference between revisions
imported>David E. Volk (stubbish) |
mNo edit summary |
||
| (13 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
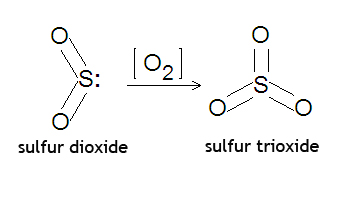
'''Sulfur dioxide''' is | {{Image|Sulfur dioxide trioxide.jpg|right|350px|Conversion of sulfur dioxide to sulfur trioxide.}} | ||
'''Sulfur dioxide''' (also spelled '''sulphur dioxide''') is a [[gas]] (SO<sub>2</sub>) whose [[molecule|molecular]] structure consists of one [[atom]] of [[sulphur]] and two atoms of [[oxygen]]. | |||
It is widely used for the manufacture of sulfuric acid, sulfurous acid and sulfite salts. It is also used as a fruit preservative and it destroys fungi and bacteria. | |||
== | == Properties and structure == | ||
It is a colorless gas with a choking odor. The structure is stabilized by utilizing a [[transargonic]] bonding pattern using one 3''d'' [[electron orbital]]. The structure includes two double bonds between sulfur and the oxygen atoms, and one lone pair of electrons on the sulfur atom. | It is a colorless gas with a choking odor. The structure is stabilized by utilizing a [[transargonic]] bonding pattern using one 3''d'' [[electron orbital]]. The structure includes two double bonds between sulfur and the oxygen atoms, and one lone pair of electrons on the sulfur atom. The length of the double bonds are short, only 1.49 Angstroms, and the O-S-O bond angle is 119.5 degrees. | ||
== | == Synthesis of sulfur dioxide == | ||
Sulfur dioxide can be produced from the oxidation of the relatively unstable sulfur monoxide. Thus, SO decomposes exothermically to yield sulfur dioxide | Sulfur dioxide can be produced from the oxidation of the relatively unstable sulfur monoxide. Thus, SO decomposes exothermically to yield sulfur dioxide | ||
| Line 14: | Line 15: | ||
It can also be produced by burning elemental sulfur or a sulfide. | It can also be produced by burning elemental sulfur or a sulfide. | ||
: S + O<sub>2</sub> &rarr SO<sub>2</sub> | : S + O<sub>2</sub> → SO<sub>2</sub> | ||
: 4FeS<sub>2</sub> + 11O<sub>2</sub> &rarr 2Fe<sub>2</sub>O<sub>3</sub> + 8SO<sub>2</sub> | : 4FeS<sub>2</sub> + 11O<sub>2</sub> → 2Fe<sub>2</sub>O<sub>3</sub> + 8SO<sub>2</sub> | ||
In the laboratory, it can be made by reacting [[sodium hydrogen sulfide]] with a strong acid, such as sulfuric acid. | In the laboratory, it can be made by reacting [[sodium hydrogen sulfide]] with a strong acid, such as sulfuric acid. | ||
: H<sub>2</sub>SO<sub>4</sub> + NaHSO<sub>3</sub> &rarr NaHSO<sub>4</sub> + H<sub>2</sub>O + SO<sub>2</sub> | : H<sub>2</sub>SO<sub>4</sub> + NaHSO<sub>3</sub> → NaHSO<sub>4</sub> + H<sub>2</sub>O + SO<sub>2</sub> | ||
The resulting gas product can be purified and dried by bubbling it through sulfuric acid. | The resulting gas product can be purified and dried by bubbling it through sulfuric acid. | ||
== | == Synthesis of other sulfur oxides and acids == | ||
Sulfur dioxide is used to make [[sulfur trioxide]], a precursor for [[sulfuric acid]], [[disulfuric acid]], [[trisulfuric acid]] and larger [[sulfur trioxide polymer]]s H(SO<sub>3</sub>)<sub>n</sub>H. | |||
: 2SO<sub>2</sub>(g) + O<sub>2</sub>(g) → 2SO<sub>3</sub>(g) | : 2SO<sub>2</sub>(g) + O<sub>2</sub>(g) → 2SO<sub>3</sub>(g) | ||
The equilibrium of this reaction is not favorable enough to be used industrially. Instead, surface chemistry using a platinum or vanadium catalyst is used for the commercial production of | The equilibrium of this reaction is not favorable enough to be used industrially. Instead, surface chemistry using a platinum or vanadium catalyst is used for the commercial production of SO<sub>3</sub>. | ||
==References== | |||
<references/>[[Category:Suggestion Bot Tag]] | |||
Latest revision as of 11:00, 23 October 2024
Sulfur dioxide (also spelled sulphur dioxide) is a gas (SO2) whose molecular structure consists of one atom of sulphur and two atoms of oxygen.
It is widely used for the manufacture of sulfuric acid, sulfurous acid and sulfite salts. It is also used as a fruit preservative and it destroys fungi and bacteria.
Properties and structure
It is a colorless gas with a choking odor. The structure is stabilized by utilizing a transargonic bonding pattern using one 3d electron orbital. The structure includes two double bonds between sulfur and the oxygen atoms, and one lone pair of electrons on the sulfur atom. The length of the double bonds are short, only 1.49 Angstroms, and the O-S-O bond angle is 119.5 degrees.
Synthesis of sulfur dioxide
Sulfur dioxide can be produced from the oxidation of the relatively unstable sulfur monoxide. Thus, SO decomposes exothermically to yield sulfur dioxide
- 16SO(g) → S8(c) + 8SO2 + 311 kJ mole-1
It can also be produced by burning elemental sulfur or a sulfide.
- S + O2 → SO2
- 4FeS2 + 11O2 → 2Fe2O3 + 8SO2
In the laboratory, it can be made by reacting sodium hydrogen sulfide with a strong acid, such as sulfuric acid.
- H2SO4 + NaHSO3 → NaHSO4 + H2O + SO2
The resulting gas product can be purified and dried by bubbling it through sulfuric acid.
Synthesis of other sulfur oxides and acids
Sulfur dioxide is used to make sulfur trioxide, a precursor for sulfuric acid, disulfuric acid, trisulfuric acid and larger sulfur trioxide polymers H(SO3)nH.
- 2SO2(g) + O2(g) → 2SO3(g)
The equilibrium of this reaction is not favorable enough to be used industrially. Instead, surface chemistry using a platinum or vanadium catalyst is used for the commercial production of SO3.