Ezetimibe: Difference between revisions
imported>David E. Volk mNo edit summary |
mNo edit summary |
||
| (4 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
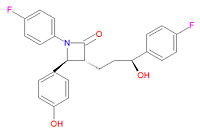
{{Image|Ezetimibe structure.jpg|right|200px|Ezetimibe.}} | |||
'''Ezetimibe''', sold under the brand names '''Ezedoc'''®, '''Zetia'''® and '''Ezetrol'''®, is an anti-[[hyperlipidemic]] medication used to lower [[cholesterol]] levels. It appears to bind to a critical mediator of cholesterol absorption, the Niemann-Pick C1-Like 1 (NPC1L1) protein on the gastrointestinal tract epithelial cells as well as in [[hepatocyte]]s. This mechanism differs from those of other classes of cholesterol-reducing compounds (HMG-CoA reductase inhibitors, bile acid sequestrants, fibric acid derivatives, and plant stanols). Ezetimibe does not inhibit cholesterol synthesis in the liver, or increase bile acid excretion but instead localizes and appears to act at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood; this distinct mechanism is complementary to that of [[Hydroxymethylglutaryl-coenzyme A reductase inhibitor|HMG-CoA reductase inhibitors]]. | '''Ezetimibe''', sold under the brand names '''Ezedoc'''®, '''Zetia'''® and '''Ezetrol'''®, is an anti-[[hyperlipidemic]] medication used to lower [[cholesterol]] levels. It appears to bind to a critical mediator of cholesterol absorption, the Niemann-Pick C1-Like 1 (NPC1L1) protein on the gastrointestinal tract epithelial cells as well as in [[hepatocyte]]s. This mechanism differs from those of other classes of cholesterol-reducing compounds (HMG-CoA reductase inhibitors, bile acid sequestrants, fibric acid derivatives, and plant stanols). Ezetimibe does not inhibit cholesterol synthesis in the liver, or increase bile acid excretion but instead localizes and appears to act at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood; this distinct mechanism is complementary to that of [[Hydroxymethylglutaryl-coenzyme A reductase inhibitor|HMG-CoA reductase inhibitors]]. | ||
Its chemical IUPAC name is (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl) azetidin-2-one and its chemical formula is C<sub>24</sub>H<sub>21</sub>F<sub>2</sub>NO<sub>3</sub>. | Its chemical IUPAC name is (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl) azetidin-2-one and its chemical formula is C<sub>24</sub>H<sub>21</sub>F<sub>2</sub>NO<sub>3</sub>. | ||
==History== | |||
Zetia brand of ezetimibe was approved for Merck and Schering-Plough by the [[Food and Drug Administration]] in the [[United States of America]] with a [http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/NewDrugApplicationNDA/ New Drug Application] (NDA) in 2002.<ref>{{FDA-Drug_Details|021445}}</ref> A generic version with a AB [[Food and Drug Administration/Catalogs/Therapeutic Equivalence Code|Therapeutic Equivalence Code]] was approved for Glenmark Generics with a [http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ Abbreviated New Drug Application] (ANDA) in 2009.<ref>{{FDA-Drug_Details|078560}}</ref> | |||
== Drug interactions == | == Drug interactions == | ||
[[Cholestyramine]] decreases the levels of ezetimibe while [[cyclosporine]] increases the effect and toxicity of ezetimibe. Ezetimibe can be taken without regard to food. | [[Cholestyramine]] decreases the levels of ezetimibe while [[cyclosporine]] increases the effect and toxicity of ezetimibe. Ezetimibe can be taken without regard to food. | ||
==Medical uses== | ==Medical uses== | ||
Ezetimibe has been studied in several [[randomized controlled trial]]s.<ref name="pmid18376000">{{cite journal |author=Kastelein JJ, Akdim F, Stroes ES, ''et al'' |title=Simvastatin with or without ezetimibe in familial hypercholesterolemia |journal=N. Engl. J. Med. |volume=358 |issue=14 |pages=1431–43 |year=2008 |month=April |pmid=18376000 |doi=10.1056/NEJMoa0800742 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=18376000&promo=ONFLNS19 |issn=}}</ref><ref name="pmid18765433">{{cite journal |author=Rossebø AB, Pedersen TR, Boman K, ''et al'' |title=Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis |journal=N. Engl. J. Med. |volume= |issue= |pages= |year=2008 |month=September |pmid=18765433 |doi=10.1056/NEJMoa0804602 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=18765433&promo=ONFLNS19 |issn=}}</ref> The ENHANCE trial found no benefit in patients with familial [[hypercholesterolemia]].<ref name="pmid18376000"/> The trial was published in 2008 although Schering-Plough completed the trial in April 2006. The SEAS trial found no benefit on [[aortic valve stenosis]]. Pending studies of ezetimibe are IMPROVE-IT (acute coronary syndrome) and SHARP (Study of Heart and Renal Protection.<ref name="pmid18314425">{{cite journal |author=Mitka M |title=Controversies surround heart drug study: questions about Vytorin and trial sponsors' conduct |journal=JAMA |volume=299 |issue=8 |pages=885–7 |year=2008 |month=February |pmid=18314425 |doi=10.1001/jama.299.8.885 |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=18314425 |issn=}}</ref> | Ezetimibe has been studied in several [[randomized controlled trial]]s.<ref name="pmid18376000">{{cite journal |author=Kastelein JJ, Akdim F, Stroes ES, ''et al'' |title=Simvastatin with or without ezetimibe in familial hypercholesterolemia |journal=N. Engl. J. Med. |volume=358 |issue=14 |pages=1431–43 |year=2008 |month=April |pmid=18376000 |doi=10.1056/NEJMoa0800742 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=18376000&promo=ONFLNS19 |issn=}}</ref><ref name="pmid18765433">{{cite journal |author=Rossebø AB, Pedersen TR, Boman K, ''et al'' |title=Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis |journal=N. Engl. J. Med. |volume= |issue= |pages= |year=2008 |month=September |pmid=18765433 |doi=10.1056/NEJMoa0804602 |url=http://content.nejm.org/cgi/pmidlookup?view=short&pmid=18765433&promo=ONFLNS19 |issn=}}</ref> The ENHANCE trial found no benefit in patients with familial [[hypercholesterolemia]].<ref name="pmid18376000"/> The trial was published in 2008 although Schering-Plough completed the trial in April 2006. The SEAS trial found no benefit on [[aortic valve stenosis]]. Pending studies of ezetimibe are IMPROVE-IT (acute coronary syndrome) and SHARP (Study of Heart and Renal Protection.<ref name="pmid18314425">{{cite journal |author=Mitka M |title=Controversies surround heart drug study: questions about Vytorin and trial sponsors' conduct |journal=JAMA |volume=299 |issue=8 |pages=885–7 |year=2008 |month=February |pmid=18314425 |doi=10.1001/jama.299.8.885 |url=http://jama.ama-assn.org/cgi/pmidlookup?view=long&pmid=18314425 |issn=}}</ref> | ||
| Line 18: | Line 22: | ||
== External Links == | == External Links == | ||
{{CZMed}} | {{CZMed}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 06:00, 15 August 2024
Ezetimibe, sold under the brand names Ezedoc®, Zetia® and Ezetrol®, is an anti-hyperlipidemic medication used to lower cholesterol levels. It appears to bind to a critical mediator of cholesterol absorption, the Niemann-Pick C1-Like 1 (NPC1L1) protein on the gastrointestinal tract epithelial cells as well as in hepatocytes. This mechanism differs from those of other classes of cholesterol-reducing compounds (HMG-CoA reductase inhibitors, bile acid sequestrants, fibric acid derivatives, and plant stanols). Ezetimibe does not inhibit cholesterol synthesis in the liver, or increase bile acid excretion but instead localizes and appears to act at the brush border of the small intestine and inhibits the absorption of cholesterol, leading to a decrease in the delivery of intestinal cholesterol to the liver. This causes a reduction of hepatic cholesterol stores and an increase in clearance of cholesterol from the blood; this distinct mechanism is complementary to that of HMG-CoA reductase inhibitors.
Its chemical IUPAC name is (3R,4S)-1-(4-fluorophenyl)-3-[(3S)-3-(4-fluorophenyl)-3-hydroxypropyl]-4-(4-hydroxyphenyl) azetidin-2-one and its chemical formula is C24H21F2NO3.
History
Zetia brand of ezetimibe was approved for Merck and Schering-Plough by the Food and Drug Administration in the United States of America with a New Drug Application (NDA) in 2002.[1] A generic version with a AB Therapeutic Equivalence Code was approved for Glenmark Generics with a Abbreviated New Drug Application (ANDA) in 2009.[2]
Drug interactions
Cholestyramine decreases the levels of ezetimibe while cyclosporine increases the effect and toxicity of ezetimibe. Ezetimibe can be taken without regard to food.
Medical uses
Ezetimibe has been studied in several randomized controlled trials.[3][4] The ENHANCE trial found no benefit in patients with familial hypercholesterolemia.[3] The trial was published in 2008 although Schering-Plough completed the trial in April 2006. The SEAS trial found no benefit on aortic valve stenosis. Pending studies of ezetimibe are IMPROVE-IT (acute coronary syndrome) and SHARP (Study of Heart and Renal Protection.[5]
Ezetimibe was a second line medication in the aggressive group (lower cholesterol and blood pressure targets) of the SANDS randomized controlled trial of Native Americans with diabetes mellitus type 2.[6] Of the 252 patients in the aggressive group, 69 received ezetimibe.[7] In a secondary analysis of the SANDS study, the carotid artery intima-media thickness regression was similar among patient in the aggressive group whether or not they received ezetimib.[7]
References
- ↑ Anonymous. Drugs@FDA for FDA Application No. 021445. U S Food and Drug Administration
- ↑ Anonymous. Drugs@FDA for FDA Application No. 078560. U S Food and Drug Administration
- ↑ 3.0 3.1 Kastelein JJ, Akdim F, Stroes ES, et al (April 2008). "Simvastatin with or without ezetimibe in familial hypercholesterolemia". N. Engl. J. Med. 358 (14): 1431–43. DOI:10.1056/NEJMoa0800742. PMID 18376000. Research Blogging.
- ↑ Rossebø AB, Pedersen TR, Boman K, et al (September 2008). "Intensive Lipid Lowering with Simvastatin and Ezetimibe in Aortic Stenosis". N. Engl. J. Med.. DOI:10.1056/NEJMoa0804602. PMID 18765433. Research Blogging.
- ↑ Mitka M (February 2008). "Controversies surround heart drug study: questions about Vytorin and trial sponsors' conduct". JAMA 299 (8): 885–7. DOI:10.1001/jama.299.8.885. PMID 18314425. Research Blogging.
- ↑ Howard BV, Roman MJ, Devereux RB, et al (April 2008). "Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: the SANDS randomized trial". JAMA 299 (14): 1678–89. DOI:10.1001/jama.299.14.1678. PMID 18398080. Research Blogging.
- ↑ 7.0 7.1 Fleg, Jerome L.; Mihriye Mete, Barbara V. Howard, Jason G. Umans, Mary J. Roman, Robert E. Ratner, Angela Silverman, James M. Galloway, Jeffrey A. Henderson, Matthew R. Weir, Charlton Wilson, Mario Stylianou, Wm. James Howard (2008-12-16). "Effect of Statins Alone Versus Statins Plus Ezetimibe on Carotid Atherosclerosis in Type 2 Diabetes: The SANDS (Stop Atherosclerosis in Native Diabetics Study) Trial". J Am Coll Cardiol 52 (25): 2198-2205. DOI:10.1016/j.jacc.2008.10.031. Retrieved on 2008-12-17. Research Blogging.
External Links
The most up-to-date information about Ezetimibe and other drugs can be found at the following sites.
- Ezetimibe - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Ezetimibe - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Ezetimibe - Detailed information from DrugBank.