Metoprolol: Difference between revisions
imported>Robert Badgett |
mNo edit summary |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 20: | Line 20: | ||
==History== | ==History== | ||
Metoprolol tartrate was developed by Novartis and received approval in the [[United States]] August 7, 1978.<ref>[http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Set_Current_Drug&ApplNo=017963&DrugName=LOPRESSOR&ActiveIngred=METOPROLOL%20TARTRATE&SponsorApplicant=NOVARTIS&ProductMktStatus=1 Drugs@FDA]. U S Food and Drug Administration</ref> | Metoprolol tartrate was developed by Novartis and received approval in the [[United States of America]] August 7, 1978.<ref>[http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Set_Current_Drug&ApplNo=017963&DrugName=LOPRESSOR&ActiveIngred=METOPROLOL%20TARTRATE&SponsorApplicant=NOVARTIS&ProductMktStatus=1 Drugs@FDA]. U S Food and Drug Administration</ref> | ||
Toprol-XL brand of metoprolol succinate was developed by [http://www.astrazenecacareers.com/content/aboutAZ/ourCompany/ourHistory/astrazeneca-our-history-corporate-evolution.asp#stuart Astra Pharmaceuticals]. Astra and Zeneca merged in 1999 to become AstraZeneca. Toprol's [[patent]] was filed on Sep 28, 1990 and approved on Jan 14, 1992 .<ref>[http://www.google.com/patents?id=shsMAAAAEBAJ Metroprolol succinate]. Google Patents.</ref> It received approval in the [[United States]] on January 10, 1992.<ref>[http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=TOPROL%2DXL Drugs@FDA]. U S Food and Drug Administration</ref> According to drugstore.com, 30 days of 100 mg pills costs $52.99 in January, 2009. | Toprol-XL brand of metoprolol succinate was developed by [http://www.astrazenecacareers.com/content/aboutAZ/ourCompany/ourHistory/astrazeneca-our-history-corporate-evolution.asp#stuart Astra Pharmaceuticals]. Astra and Zeneca merged in 1999 to become AstraZeneca. Toprol's [[patent]] was filed on Sep 28, 1990 and approved on Jan 14, 1992 .<ref>[http://www.google.com/patents?id=shsMAAAAEBAJ Metroprolol succinate]. Google Patents.</ref> It received approval in the [[United States of America]] on January 10, 1992.<ref>[http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Overview&DrugName=TOPROL%2DXL Drugs@FDA]. U S Food and Drug Administration</ref> According to drugstore.com, 30 days of 100 mg pills costs $52.99 in January, 2009. | ||
Generic metoprolol succinate was developed by Sandoz and received approval in the [[United States]] on July 31, 2006. According to drugstore.com, 30 days of 100 mg pills costs $25.99 in January, 2009. | Generic metoprolol succinate was developed by Sandoz and received approval in the [[United States of America]] on July 31, 2006. According to drugstore.com, 30 days of 100 mg pills costs $25.99 in January, 2009. | ||
Astrazenaca and the manufacturer of generic metoprolol succinate have engaged in a lawsuit.<ref name="urlwww.cafc.uscourts.gov">{{cite web |url=http://www.cafc.uscourts.gov/opinions/06-1254.pdf |title=United States Court of Appeals for the Federal Circuit. 2006-1254. IN RE METOPROLOL SUCCINATE PATENT LITIGATION |author=United States Court of Appeals for the Federal Circuit |authorlink=http://www.cafc.uscourts.gov/ |coauthors= |date=July 23, 2007 |format=pdf |work= |publisher= |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=2009-01-11}}</ref> | Astrazenaca and the manufacturer of generic metoprolol succinate have engaged in a lawsuit.<ref name="urlwww.cafc.uscourts.gov">{{cite web |url=http://www.cafc.uscourts.gov/opinions/06-1254.pdf |title=United States Court of Appeals for the Federal Circuit. 2006-1254. IN RE METOPROLOL SUCCINATE PATENT LITIGATION |author=United States Court of Appeals for the Federal Circuit |authorlink=http://www.cafc.uscourts.gov/ |coauthors= |date=July 23, 2007 |format=pdf |work= |publisher= |pages= |language= |archiveurl= |archivedate= |quote= |accessdate=2009-01-11}}</ref> | ||
| Line 36: | Line 36: | ||
It is metabolized by [[cytochrome P-450]] [http://www.ncbi.nlm.nih.gov/sites/entrez/?db=gene&cmd=Retrieve&dopt=summary&list_uids=1565 2D6] [[allele]] and so may have drug interactions<ref name="pmid18452693">{{cite journal |author=Onalan O, Cumurcu BE, Bekar L |title=Complete atrioventricular block associated with concomitant use of metoprolol and paroxetine |journal=Mayo Clin. Proc. |volume=83 |issue=5 |pages=595–9 |year=2008 |month=May |pmid=18452693 |doi= |url=http://www.mayoclinicproceedings.com/Abstract.asp?AID=4681&Abst=Abstract&UID= |issn=}}</ref> and inherited variations in metabolism.<ref name="pmid16220080">{{cite journal |author=Nozawa T, Taguchi M, Tahara K, ''et al'' |title=Influence of CYP2D6 genotype on metoprolol plasma concentration and beta-adrenergic inhibition during long-term treatment: a comparison with bisoprolol |journal=J. Cardiovasc. Pharmacol. |volume=46 |issue=5 |pages=713–20 |year=2005 |month=November |pmid=16220080 |doi= |url=http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0160-2446&volume=46&issue=5&spage=713 |issn=}}</ref> | It is metabolized by [[cytochrome P-450]] [http://www.ncbi.nlm.nih.gov/sites/entrez/?db=gene&cmd=Retrieve&dopt=summary&list_uids=1565 2D6] [[allele]] and so may have drug interactions<ref name="pmid18452693">{{cite journal |author=Onalan O, Cumurcu BE, Bekar L |title=Complete atrioventricular block associated with concomitant use of metoprolol and paroxetine |journal=Mayo Clin. Proc. |volume=83 |issue=5 |pages=595–9 |year=2008 |month=May |pmid=18452693 |doi= |url=http://www.mayoclinicproceedings.com/Abstract.asp?AID=4681&Abst=Abstract&UID= |issn=}}</ref> and inherited variations in metabolism.<ref name="pmid16220080">{{cite journal |author=Nozawa T, Taguchi M, Tahara K, ''et al'' |title=Influence of CYP2D6 genotype on metoprolol plasma concentration and beta-adrenergic inhibition during long-term treatment: a comparison with bisoprolol |journal=J. Cardiovasc. Pharmacol. |volume=46 |issue=5 |pages=713–20 |year=2005 |month=November |pmid=16220080 |doi= |url=http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0160-2446&volume=46&issue=5&spage=713 |issn=}}</ref> | ||
Individuals with reduced metabolism genotypes report [[ | Individuals with reduced metabolism genotypes report increased [[drug toxicity]] from metoprolol<ref name="pmid12386645">{{cite journal| author=Wuttke H, Rau T, Heide R, Bergmann K, Böhm M, Weil J et al.| title=Increased frequency of cytochrome P450 2D6 poor metabolizers among patients with metoprolol-associated adverse effects. | journal=Clin Pharmacol Ther | year= 2002 | volume= 72 | issue= 4 | pages= 429-37 | pmid=12386645 | ||
| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&email=badgett@uthscdsa.edu&retmode=ref&cmd=prlinks&id=12386645 | doi=10.1067/mcp.2002.127111 }} <!--Formatted by http://sumsearch.uthscsa.edu/cite/--></ref> and require more adjustments in dose<ref name="pmid15735607">{{cite journal| author=Terra SG, Pauly DF, Lee CR, Patterson JH, Adams KF, Schofield RS et al.| title=beta-Adrenergic receptor polymorphisms and responses during titration of metoprolol controlled release/extended release in heart failure. | journal=Clin Pharmacol Ther | year= 2005 | volume= 77 | issue= 3 | pages= 127-37 | pmid=15735607 | | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&email=badgett@uthscdsa.edu&retmode=ref&cmd=prlinks&id=12386645 | doi=10.1067/mcp.2002.127111 }} <!--Formatted by http://sumsearch.uthscsa.edu/cite/--></ref> and require more adjustments in dose<ref name="pmid15735607">{{cite journal| author=Terra SG, Pauly DF, Lee CR, Patterson JH, Adams KF, Schofield RS et al.| title=beta-Adrenergic receptor polymorphisms and responses during titration of metoprolol controlled release/extended release in heart failure. | journal=Clin Pharmacol Ther | year= 2005 | volume= 77 | issue= 3 | pages= 127-37 | pmid=15735607 | ||
| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&email=badgett@uthscdsa.edu&retmode=ref&cmd=prlinks&id=15735607 | doi=10.1016/j.clpt.2004.10.006 }} <!--Formatted by http://sumsearch.uthscsa.edu/cite/--></ref>. However, when a sufficiently long period of time is used to titrate the dosage, adverse effects are not increased.<ref name="pmid16198657">{{cite journal| author=Fux R, Mörike K, Pröhmer AM, Delabar U, Schwab M, Schaeffeler E et al.| title=Impact of CYP2D6 genotype on adverse effects during treatment with metoprolol: a prospective clinical study. | journal=Clin Pharmacol Ther | year= 2005 | volume= 78 | issue= 4 | pages= 378-87 | pmid=16198657 | | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=clinical.uthscsa.edu/cite&email=badgett@uthscdsa.edu&retmode=ref&cmd=prlinks&id=15735607 | doi=10.1016/j.clpt.2004.10.006 }} <!--Formatted by http://sumsearch.uthscsa.edu/cite/--></ref>. However, when a sufficiently long period of time is used to titrate the dosage, adverse effects are not increased.<ref name="pmid16198657">{{cite journal| author=Fux R, Mörike K, Pröhmer AM, Delabar U, Schwab M, Schaeffeler E et al.| title=Impact of CYP2D6 genotype on adverse effects during treatment with metoprolol: a prospective clinical study. | journal=Clin Pharmacol Ther | year= 2005 | volume= 78 | issue= 4 | pages= 378-87 | pmid=16198657 | ||
| Line 52: | Line 52: | ||
==References== | ==References== | ||
<references/> | <references/>[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:01, 18 September 2024
|
| |||||||
| metoprolol | |||||||
| |||||||
| Uses: | hypertension, angina | ||||||
| Properties: | adrenergic beta-antagonist | ||||||
| Hazards: | |||||||
| |||||||
In medicine, metoprolol is a adrenergic beta-antagonist medication that is a "selective adrenergic beta-1-blocking agent with no stimulatory action. It's binding to plasma albumin is weaker than alprenolol and it may be useful in angina pectoris, hypertension, or cardiac arrhythmias." It is also used for heart failure.[1]
History
Metoprolol tartrate was developed by Novartis and received approval in the United States of America August 7, 1978.[2]
Toprol-XL brand of metoprolol succinate was developed by Astra Pharmaceuticals. Astra and Zeneca merged in 1999 to become AstraZeneca. Toprol's patent was filed on Sep 28, 1990 and approved on Jan 14, 1992 .[3] It received approval in the United States of America on January 10, 1992.[4] According to drugstore.com, 30 days of 100 mg pills costs $52.99 in January, 2009.
Generic metoprolol succinate was developed by Sandoz and received approval in the United States of America on July 31, 2006. According to drugstore.com, 30 days of 100 mg pills costs $25.99 in January, 2009.
Astrazenaca and the manufacturer of generic metoprolol succinate have engaged in a lawsuit.[5]
Chemistry
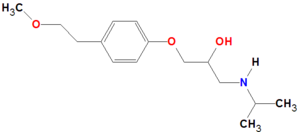
The IUPAC name of metroprolol is:
- (±)-1-(Isopropylamino)-3-[p-(2-methoxyethyl)phenoxy]-2-propanol (according to Dailymed)
- 1-[4-(2-methoxyethyl)phenoxy]-3-(propan-2-ylamino)propan-2-ol (according to DrugBank)[6]
Metabolism
It is metabolized by cytochrome P-450 2D6 allele and so may have drug interactions[7] and inherited variations in metabolism.[8]
Individuals with reduced metabolism genotypes report increased drug toxicity from metoprolol[9] and require more adjustments in dose[10]. However, when a sufficiently long period of time is used to titrate the dosage, adverse effects are not increased.[11][12]
Dosage
For healthy adults, the starting dose is 25 to 100 mg daily in single or divided doses and the maximum dose is 400 to 450 mg/day.
Efficacy
Metoprolol can benefit patients with heart failure.[13]
External links
The most up-to-date information about Metoprolol and other drugs can be found at the following sites.
- Metoprolol - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Metoprolol - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Metoprolol - Detailed information from DrugBank.
References
- ↑ Anonymous (2024), Metoprolol (English). Medical Subject Headings. U.S. National Library of Medicine.
- ↑ Drugs@FDA. U S Food and Drug Administration
- ↑ Metroprolol succinate. Google Patents.
- ↑ Drugs@FDA. U S Food and Drug Administration
- ↑ [States Court of Appeals for the Federal Circuit] (July 23, 2007). United States Court of Appeals for the Federal Circuit. 2006-1254. IN RE METOPROLOL SUCCINATE PATENT LITIGATION (pdf). Retrieved on 2009-01-11.
- ↑ Showing Metoprolol (DB00264). DrugBank. Retrieved on 2009-06-20.
- ↑ Onalan O, Cumurcu BE, Bekar L (May 2008). "Complete atrioventricular block associated with concomitant use of metoprolol and paroxetine". Mayo Clin. Proc. 83 (5): 595–9. PMID 18452693. [e]
- ↑ Nozawa T, Taguchi M, Tahara K, et al (November 2005). "Influence of CYP2D6 genotype on metoprolol plasma concentration and beta-adrenergic inhibition during long-term treatment: a comparison with bisoprolol". J. Cardiovasc. Pharmacol. 46 (5): 713–20. PMID 16220080. [e]
- ↑ Wuttke H, Rau T, Heide R, Bergmann K, Böhm M, Weil J et al. (2002). "Increased frequency of cytochrome P450 2D6 poor metabolizers among patients with metoprolol-associated adverse effects.". Clin Pharmacol Ther 72 (4): 429-37. DOI:10.1067/mcp.2002.127111. PMID 12386645. Research Blogging.
- ↑ Terra SG, Pauly DF, Lee CR, Patterson JH, Adams KF, Schofield RS et al. (2005). "beta-Adrenergic receptor polymorphisms and responses during titration of metoprolol controlled release/extended release in heart failure.". Clin Pharmacol Ther 77 (3): 127-37. DOI:10.1016/j.clpt.2004.10.006. PMID 15735607. Research Blogging.
- ↑ Fux R, Mörike K, Pröhmer AM, Delabar U, Schwab M, Schaeffeler E et al. (2005). "Impact of CYP2D6 genotype on adverse effects during treatment with metoprolol: a prospective clinical study.". Clin Pharmacol Ther 78 (4): 378-87. DOI:10.1016/j.clpt.2005.07.004. PMID 16198657. Research Blogging.
- ↑ Zineh I, Beitelshees AL, Gaedigk A, Walker JR, Pauly DF, Eberst K et al. (2004). "Pharmacokinetics and CYP2D6 genotypes do not predict metoprolol adverse events or efficacy in hypertension.". Clin Pharmacol Ther 76 (6): 536-44. DOI:10.1016/j.clpt.2004.08.020. PMID 15592325. Research Blogging.
- ↑ Waagstein F, Bristow MR, Swedberg K, et al (December 1993). "Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metoprolol in Dilated Cardiomyopathy (MDC) Trial Study Group". Lancet 342 (8885): 1441–6. PMID 7902479. [e]