Naegleria fowleri: Difference between revisions
imported>Nicole Perrotta No edit summary |
mNo edit summary |
||
| (83 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{Taxobox | color = khaki | {{Taxobox | color = khaki | ||
| name = ''Naegleria fowleri'' | | name = ''Naegleria fowleri'' | ||
| Line 16: | Line 14: | ||
| species = '''N. fowleri''' | | species = '''N. fowleri''' | ||
| binomial = ''Naegleria fowleri'' | | binomial = ''Naegleria fowleri'' | ||
| binomial_authority = [[Carter]] (1970) | | binomial_authority = [[Carter]] (1970'')'' | ||
}} | }} | ||
==Description and significance== | ==Description and significance== | ||
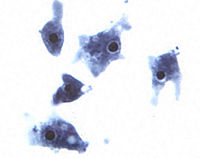
Naegleria fowleri (commonly known as the “brain-eating amoeba) is a thermophilic free-living amoeba found in moist environments (25-35 degrees Celsius) including warm fresh water, soil | {{Image|Naegleria fowleri trophozoites.jpg|left|200px|''Naegleria fowleri'' trophozoites cultured from cerebrospinal fluid.}} | ||
''Naegleria fowleri'' (commonly known as the “brain-eating amoeba) is a thermophilic free-living amoeba found in moist environments (25-35 degrees Celsius) including warm fresh water, geothermal springs, soil and sewage. It is not found in salt water. N. fowleri is the causative agent of primary amoebic meningoencephalitis (PAM), a rare but nearly always fatal disease of the central nervous system<ref name=cdc>[http://www.cdc.gov/ncidod/dpd/parasites/Naegleria/factsht_naegleria.htm Center For Disease Control and Prevention. "''Naegleria'' Infection" 2 May 2008]</ref>. Cases of PAM resulting from infection with ''Naegleria fowleri'' have been reported in over fifteen countries in Africa, Asia, Europe, and North and South America<ref name=awqc>[http://www.awqc.com.au/NR/rdonlyres/2732DDCF-42C2-47FC-97A1-AD4C718656CD/0/Amoebae.pdf "Pathogenic Free-Living Amoebae" Australian Water Quality Centre 17 Feb 2009]</ref>. ''Naegleria fowleri'' belongs to the Percolozoa phylum and can exist in different forms including trophozoite, flagellate, and encysted.<ref>{{cite web|date=11 September 2013|title=Naegleria fowleri - Primary Amebic Meningoencephalitis (PAM)|url=http://www.cdc.gov/parasites/naegleria/pathogen.html|publisher=Centers for Disease Control and Prevention|accessdate=15 October 2013}}</ref> | |||
==Genome structure== | ==Genome structure== | ||
Although the genome of N. fowleri has yet not been sequenced, several ways of distinguishing between N. fowleri and non-pathogenic species of Naegleria by PCR have been developed. Since timely diagnosis of primary amoebic meningoencephalitis is imperative for survival, rapid detection of N. fowleri is crucial. A real-time, multiplex PCR assay was recently developed to identify the DNA of N. fowleri from samples of cerebrospinal fluid. The identification can usually be done within five hours of entering the laboratory<ref>[http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=167479 Kilvington, S; J Beeching. "Identification and Epidemiological Typing of ''Naegleria fowleri'' with DNA Probes" Appl. Environ. Microbiology. 1995 Volume 61, pgs. 2071-2078 June 1995]</ref><ref name=microbewiki>[http://microbewiki.kenyon.edu/index.php/Naegleria MicrobeWiki "Naegleria" 5 December 2008]</ref>. | |||
==Life Cycle== | |||
''Naegleria fowleri'' is an amoeba with a three-stage life cycle. | |||
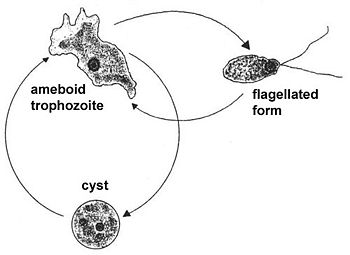
{{Image|forms.jpg|left|350px|Different Forms in Life Cycle of ''Naegleria fowleri''.}} | |||
The trophozoite is the reproductive and feeding stage of the N. fowleri lifecycle. During this stage N. fowleri reproduces via binary fission. This is the only stage of its life cycle during which it reproduces. In this form it measures 10-25 µm in diameter and moves by way of its lobopodium which project from the surface of the bacteria<ref name=scidirect>[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T5D-4TPGNFD-1&_user=699469&_coverDate=11%2F01%2F2008&_alid=905253846&_rdoc=5&_fmt=high&_orig=search&_cdi=5000&_sort=d&_st=4&_docanchor=&_ct=106&_acct=C000039278&_version=1&_urlVersion=0&_userid=699469&md5=8c3f62c6856328f201b651512f78e30e Visvesvara, GS PhD; FL Schuster, PhD. "Opportunistic Free-Living Amebae, Part II" "Clinical Microbiology Newsletter" Volume 30, No. 21 1 Nov 2008 ]</ref>. The lobopodium allow the trophozoites to ingest their food, mostly other bacteria or yeast. Once N. fowleri infects the human nervous system, it enters the trophozoite stage and feeds on red and white blood cells causing tissue damage and eventually necrosis<ref name=eMedicine>[http://emedicine.medscape.com/article/223910-overview Parija, Subhash Chandra, MBBS, MD, PhD; Michael Stuart Bronze, MD; Barnett Gibbs, MD; Diane H Johnson, MD. “Naegleria Infection” ''eMedicine''. 12 Feb 2009 ]</ref>. | |||
== | When the trophozoite is exposed to water, it may enter the temporary flagellate stage. The flagellate stage of ''Naegleria fowleri'' is temporary and allows for rapid movement or swimming. During this stage, N. fowleri is pear-shaped and has two flagella attached at its broader end. The flagellate stage is when the ameba can infect an individual by swimming up the nasal cavity, where it enters the trophozoite form. The amoeba does not feed or divide during its flagellate stage<ref name=eMedicine /><ref name=suite101>[http://human-infections.suite101.com/article.cfm/naegleria_fowleri_deadly_amoeba Drisdelle, Rosemary. "''Naegleria fowleri'' - Deadly Amoeba suite101.com 11 Sep 2007]</ref>. | ||
When ''Naegleria fowleri'' is exposed to harsh conditions such as aridity or food shortage it enters the cystic, or resistant, stage. The encysted amoeba is round and measures 7-10 µm in diameter. It is protected by a smooth double layered wall that is 1 µm thick<ref name=scidirect />. | |||
When Naegleria fowleri is exposed to harsh conditions such as aridity or food shortage it enters the cystic, or resistant, stage. The encysted amoeba is round and measures 7-10 µm in diameter. It is protected by a smooth double layered wall that is 1 µm thick. | |||
==Ecology== | ==Ecology== | ||
''Naegleria fowleri'' is a thermophilic protist and can be found in many moist or aqueous environments, including freshwater lakes, rivers, ponds, sewage, hot springs, moist soil, and thermally polluted bodies of water. It can also be found in swimming pools or stagnant water that has not been cared for by chlorination. It grows fastest at 42 degrees Celsius, and is able to survive and multiply in mammalian body temperatures<ref name=eMedicine />. Most N. fowleri infections in the United States occur in the summertime in the Southern tier states, when its growth and reproduction is favored by the warm temperatures. N. fowleri is sensitive to environmental conditions such as desiccation, pH extremes, and high salinity<ref name=cdc /><ref name=awqc />. | |||
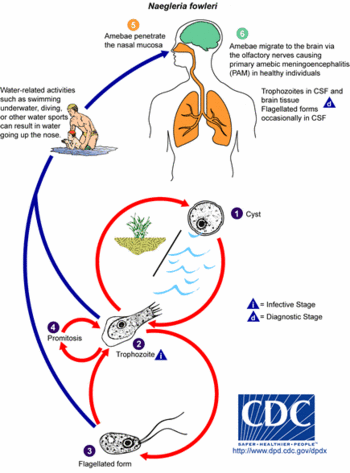
Naegleria fowleri | {{Image|Naegleria fowleri Life Cycle.gif|right|350px|Life Cycle of ''Naegleria fowleri''.}} | ||
==Pathology== | ==Pathology== | ||
''Naegleria fowleri'' can cause the disease primary meningoencephalitis (PAM) in humans or animals. Initial symptoms of PAM are closely related to those of bacterial meningitis. Initial symptoms include headache, fever, changes in taste or smell, stiff neck, nausea and vomiting. Later symptoms include confusion, lethargy, seizures and hallucinations. These symptoms are almost always accompanied by rapid onset of coma and death within 3-5 days after symptoms first occur<ref name=eMedicine /><ref name=scidirect />. | |||
N. fowleri infects its victims by entering the CNS through the nose, usually while the victims are swimming in warm freshwater lakes or hot springs. The amoebas, which are in flagellate form in the water, swim into nasal cavitiy where they enter the trophozoite (“feeding”) form. They crawl up the olfactory mucosa, along nerve fibers on the floor of the cranium, and through the cribiform plate into the brain. The glucose and protein present in the cerebrospinal fluid of the brain support the growth and rapid multiplication of the amoebas. N. fowleri feeds on red blood cells and white blood cells in the brain resulting in hemorrhaging and necrosis<ref name=scidirect />. | |||
N. fowleri infects its victims by entering the CNS through the nose, usually while the victims are swimming in warm freshwater lakes or hot springs. The amoebas, which are in flagellate form in the water, swim into nasal cavitiy where they enter the trophozoite (“feeding”) form. They crawl up the olfactory mucosa, along nerve fibers on the floor of the cranium, and through the cribiform plate into the brain. The glucose and protein present in the cerebrospinal fluid of the brain support the growth and rapid multiplication of the amoebas. N. fowleri feeds on red blood cells and white blood cells in the brain resulting in hemorrhaging and necrosis. | |||
The mortality rate of PAM is over 95% and in the rare cases of survival early detection is key. One of the few survivors of PAM was treated early and aggressively with intravenous and intrathecal amphotericin B, miconazole, and oral rifampin<ref name=survival>[http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WN2-4T7W3M4-5&_user=699469&_coverDate=12%2F31%2F2008&_alid=905253846&_rdoc=4&_fmt=high&_orig=search&_cdi=6950&_sort=d&_st=4&_docanchor=&_ct=106&_acct=C000039278&_version=1&_urlVersion=0&_userid=699469&md5=02a61b8a4e8161c7d63be5fcc13fe9b3&errMsg=1 Alisky, Joseph Martin. "Survival of ''Naegleria fowleri'' Primary Amebic Meningocephalitis (PAM) could be Improved with an Intensive Multi-Route Chemo- and Biotherapeutic Regimen" "Medical Hypotheses" 2008 Volume 71, pgs. 969-971 26 June 2008 ]</ref>. | |||
==Application to Biotechnology== | ==Application to Biotechnology== | ||
The use of ''Naegleria fowleri'' in current biotechnology is limited to the development of tests for its presence in infected individuals and in hazardous bodies of water or water supplies<ref name=microbewiki />. | |||
The use of Naegleria fowleri in current biotechnology is limited to the development of tests for its presence in infected individuals and in hazardous bodies of water or water supplies. | |||
==Current Research== | ==Current Research== | ||
"'''Contact-Independent Cell Death of Human Microglial Cells Due to Pathogenic ''Naegleria fowleri'' Trophozoites'''" | |||
== | This study was conducted to determine if target cell death in humans infected with primary amebic meningoencephalitis (PAM) is dependent on the contact-dependent method of phagocytosis by ''Naegleria fowleri.'' U87MG human microglial cells were co-cultured with N. fowleri trophozoites for 20 minutes, 2 hours, and 4 hours in a non-contact system. The microglial cells reduced in number and showed morphological changes including cell membrane destruction. Using flourescence-activated cell sorter (FACS) analysis, it was determined that the the number of apoptotic cells increased by 16% in comparison with the control. In the non-contact system the cytoxicity of the ''Naegleria fowleri'' trophozoite against the target U87MG human microglial cells was 40.5% for 30 minutes, 44.2% for 2 hours, and 45.6% for 4 hours. This was compared to the cytoxicity against the target U87MG human microglial cells of the non-pathogenic species ''Naegleria gruberi'' in a non-contact system which showed 10.2, 12.4, and 13.2% cytoxicity, respectively. The results of this study suggest that molecules released by N. fowleri in a non-contact manner as well as phagocystosis in a contact-dependent manner may induce cell death in the host microglial cells <ref>[http://www.ncbi.nlm.nih.gov/pubmed/19127326?ordinalpos=7&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum Kim JH, D Kim, HJ Shin. "Contact-Independent Cell Death of Human Microglial Cells Due to Pathogenic Naegleria fowleri Trophozoites.” Korean Journal of Parasitology. 2008 Dec; 46(4):217-21. Epub 2008 Dec 20.]</ref>. | ||
"'''Survival of ''Naegleria fowleri'' Primary Amebic Meningocephalitis (PAM) Could be Improved with an Intensive Multi-Route Chemo- and Biotherapeutic Regimen'''" | |||
Primary amebic meningocephalitis has emerged as a perilous public health threat in recent years. The mortality rate of the parasitic disease PAM is nearly 95%. The few survivors of PAM were treating intravenously and intrathecally with amphotericin coupled with other drugs including dexamathasone, diflucan, chloramphenicol, and rifampin. This study hypothesizes that survival after infection with ''Naegleria fowleri'' could be improved with combined intrathecal, intranasal and intravenous amphotericin, diflucan, and rifampin, with aduvant intravenous chloramphenicol, muramyl dipeptide, azithromycin, minocycline and linezolid, intramuscular trifluoperazine, intranasal Cry1C protoxin and intrathecal anti-Naegleria immune globulin and dexamethasone. The rationale for this hypothesis is that introducing these medications intranasally, intravenously and intrathecally would target the primary sites of infection as well as those sites it has spread to. Intrathecal dexamethasone should mitigate cerebral edema, one of the primary causes of death in PAM. Azithromycin and minocycline have appeared to aid amphotericin in killing N. fowleri in animal models. The other drugs, as shown in animal models, appear to have synergistic effects as well. The approach to PAM would be similar to the aggressive approach of chemotherapy for tuberculosis and cancer treatment, with multidrug therapy to assure complete elimination of the infection. The hypothesis has not yet been tested<ref name=survival />. | |||
"''' Use of Organs for Transplantation From a Donor with Primary Meningoencephalitis Due to ''Naegleria fowleri'''''" | |||
Because of the infectious nature of the parasite ''Naegleria fowleri,'' causative agent of primary amebic meningoencephalitis (PAM), victims of PAM were originally ruled out as organ donors. Once it was realized that the infection is limited to the central nervous system, organ donation was no longer ruled out. The study tells of the successful organ donation from a 12-year old male donor dying of PAM. Kidneys, pancreas, lungs and liver were retrieved from the male and transplanted. There is no evidence of posttransplant infectious complications. The results of this study suggest that this brain infection does not prevent successful organ donation<ref>[http://www.ncbi.nlm.nih.gov/pubmed/18444934?ordinalpos=18&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultReportPanel.Pubmed_RVDocSum Bennett, WM, JF Nespral, MW Rosson, KM McEvoy. "Use of Organs for Transplantation From a Donor with Primary Meningoencephalitis Due to Naegleria fowleri.” American Journal of Transplantation. 2008 Jun; 8(6): 1334-5. Epub 2008 Apr 29.]</ref>. | |||
==References== | |||
{{reflist}}[[Category:Suggestion Bot Tag]] | |||
[ | |||
Latest revision as of 06:01, 23 September 2024
| Naegleria fowleri | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
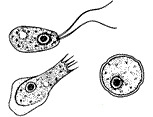 Different stages of Naegleria fowleri
| ||||||||||||||||
| Scientific classification | ||||||||||||||||
| ||||||||||||||||
| Binomial name | ||||||||||||||||
| Naegleria fowleri Carter (1970) |
Description and significance
Naegleria fowleri (commonly known as the “brain-eating amoeba) is a thermophilic free-living amoeba found in moist environments (25-35 degrees Celsius) including warm fresh water, geothermal springs, soil and sewage. It is not found in salt water. N. fowleri is the causative agent of primary amoebic meningoencephalitis (PAM), a rare but nearly always fatal disease of the central nervous system[1]. Cases of PAM resulting from infection with Naegleria fowleri have been reported in over fifteen countries in Africa, Asia, Europe, and North and South America[2]. Naegleria fowleri belongs to the Percolozoa phylum and can exist in different forms including trophozoite, flagellate, and encysted.[3]
Genome structure
Although the genome of N. fowleri has yet not been sequenced, several ways of distinguishing between N. fowleri and non-pathogenic species of Naegleria by PCR have been developed. Since timely diagnosis of primary amoebic meningoencephalitis is imperative for survival, rapid detection of N. fowleri is crucial. A real-time, multiplex PCR assay was recently developed to identify the DNA of N. fowleri from samples of cerebrospinal fluid. The identification can usually be done within five hours of entering the laboratory[4][5].
Life Cycle
Naegleria fowleri is an amoeba with a three-stage life cycle.
The trophozoite is the reproductive and feeding stage of the N. fowleri lifecycle. During this stage N. fowleri reproduces via binary fission. This is the only stage of its life cycle during which it reproduces. In this form it measures 10-25 µm in diameter and moves by way of its lobopodium which project from the surface of the bacteria[6]. The lobopodium allow the trophozoites to ingest their food, mostly other bacteria or yeast. Once N. fowleri infects the human nervous system, it enters the trophozoite stage and feeds on red and white blood cells causing tissue damage and eventually necrosis[7].
When the trophozoite is exposed to water, it may enter the temporary flagellate stage. The flagellate stage of Naegleria fowleri is temporary and allows for rapid movement or swimming. During this stage, N. fowleri is pear-shaped and has two flagella attached at its broader end. The flagellate stage is when the ameba can infect an individual by swimming up the nasal cavity, where it enters the trophozoite form. The amoeba does not feed or divide during its flagellate stage[7][8].
When Naegleria fowleri is exposed to harsh conditions such as aridity or food shortage it enters the cystic, or resistant, stage. The encysted amoeba is round and measures 7-10 µm in diameter. It is protected by a smooth double layered wall that is 1 µm thick[6].
Ecology
Naegleria fowleri is a thermophilic protist and can be found in many moist or aqueous environments, including freshwater lakes, rivers, ponds, sewage, hot springs, moist soil, and thermally polluted bodies of water. It can also be found in swimming pools or stagnant water that has not been cared for by chlorination. It grows fastest at 42 degrees Celsius, and is able to survive and multiply in mammalian body temperatures[7]. Most N. fowleri infections in the United States occur in the summertime in the Southern tier states, when its growth and reproduction is favored by the warm temperatures. N. fowleri is sensitive to environmental conditions such as desiccation, pH extremes, and high salinity[1][2].
Pathology
Naegleria fowleri can cause the disease primary meningoencephalitis (PAM) in humans or animals. Initial symptoms of PAM are closely related to those of bacterial meningitis. Initial symptoms include headache, fever, changes in taste or smell, stiff neck, nausea and vomiting. Later symptoms include confusion, lethargy, seizures and hallucinations. These symptoms are almost always accompanied by rapid onset of coma and death within 3-5 days after symptoms first occur[7][6].
N. fowleri infects its victims by entering the CNS through the nose, usually while the victims are swimming in warm freshwater lakes or hot springs. The amoebas, which are in flagellate form in the water, swim into nasal cavitiy where they enter the trophozoite (“feeding”) form. They crawl up the olfactory mucosa, along nerve fibers on the floor of the cranium, and through the cribiform plate into the brain. The glucose and protein present in the cerebrospinal fluid of the brain support the growth and rapid multiplication of the amoebas. N. fowleri feeds on red blood cells and white blood cells in the brain resulting in hemorrhaging and necrosis[6].
The mortality rate of PAM is over 95% and in the rare cases of survival early detection is key. One of the few survivors of PAM was treated early and aggressively with intravenous and intrathecal amphotericin B, miconazole, and oral rifampin[9].
Application to Biotechnology
The use of Naegleria fowleri in current biotechnology is limited to the development of tests for its presence in infected individuals and in hazardous bodies of water or water supplies[5].
Current Research
"Contact-Independent Cell Death of Human Microglial Cells Due to Pathogenic Naegleria fowleri Trophozoites"
This study was conducted to determine if target cell death in humans infected with primary amebic meningoencephalitis (PAM) is dependent on the contact-dependent method of phagocytosis by Naegleria fowleri. U87MG human microglial cells were co-cultured with N. fowleri trophozoites for 20 minutes, 2 hours, and 4 hours in a non-contact system. The microglial cells reduced in number and showed morphological changes including cell membrane destruction. Using flourescence-activated cell sorter (FACS) analysis, it was determined that the the number of apoptotic cells increased by 16% in comparison with the control. In the non-contact system the cytoxicity of the Naegleria fowleri trophozoite against the target U87MG human microglial cells was 40.5% for 30 minutes, 44.2% for 2 hours, and 45.6% for 4 hours. This was compared to the cytoxicity against the target U87MG human microglial cells of the non-pathogenic species Naegleria gruberi in a non-contact system which showed 10.2, 12.4, and 13.2% cytoxicity, respectively. The results of this study suggest that molecules released by N. fowleri in a non-contact manner as well as phagocystosis in a contact-dependent manner may induce cell death in the host microglial cells [10].
"Survival of Naegleria fowleri Primary Amebic Meningocephalitis (PAM) Could be Improved with an Intensive Multi-Route Chemo- and Biotherapeutic Regimen"
Primary amebic meningocephalitis has emerged as a perilous public health threat in recent years. The mortality rate of the parasitic disease PAM is nearly 95%. The few survivors of PAM were treating intravenously and intrathecally with amphotericin coupled with other drugs including dexamathasone, diflucan, chloramphenicol, and rifampin. This study hypothesizes that survival after infection with Naegleria fowleri could be improved with combined intrathecal, intranasal and intravenous amphotericin, diflucan, and rifampin, with aduvant intravenous chloramphenicol, muramyl dipeptide, azithromycin, minocycline and linezolid, intramuscular trifluoperazine, intranasal Cry1C protoxin and intrathecal anti-Naegleria immune globulin and dexamethasone. The rationale for this hypothesis is that introducing these medications intranasally, intravenously and intrathecally would target the primary sites of infection as well as those sites it has spread to. Intrathecal dexamethasone should mitigate cerebral edema, one of the primary causes of death in PAM. Azithromycin and minocycline have appeared to aid amphotericin in killing N. fowleri in animal models. The other drugs, as shown in animal models, appear to have synergistic effects as well. The approach to PAM would be similar to the aggressive approach of chemotherapy for tuberculosis and cancer treatment, with multidrug therapy to assure complete elimination of the infection. The hypothesis has not yet been tested[9].
" Use of Organs for Transplantation From a Donor with Primary Meningoencephalitis Due to Naegleria fowleri"
Because of the infectious nature of the parasite Naegleria fowleri, causative agent of primary amebic meningoencephalitis (PAM), victims of PAM were originally ruled out as organ donors. Once it was realized that the infection is limited to the central nervous system, organ donation was no longer ruled out. The study tells of the successful organ donation from a 12-year old male donor dying of PAM. Kidneys, pancreas, lungs and liver were retrieved from the male and transplanted. There is no evidence of posttransplant infectious complications. The results of this study suggest that this brain infection does not prevent successful organ donation[11].
References
- ↑ 1.0 1.1 Center For Disease Control and Prevention. "Naegleria Infection" 2 May 2008
- ↑ 2.0 2.1 "Pathogenic Free-Living Amoebae" Australian Water Quality Centre 17 Feb 2009
- ↑ Naegleria fowleri - Primary Amebic Meningoencephalitis (PAM). Centers for Disease Control and Prevention (11 September 2013). Retrieved on 15 October 2013.
- ↑ Kilvington, S; J Beeching. "Identification and Epidemiological Typing of Naegleria fowleri with DNA Probes" Appl. Environ. Microbiology. 1995 Volume 61, pgs. 2071-2078 June 1995
- ↑ 5.0 5.1 MicrobeWiki "Naegleria" 5 December 2008
- ↑ 6.0 6.1 6.2 6.3 Visvesvara, GS PhD; FL Schuster, PhD. "Opportunistic Free-Living Amebae, Part II" "Clinical Microbiology Newsletter" Volume 30, No. 21 1 Nov 2008
- ↑ 7.0 7.1 7.2 7.3 Parija, Subhash Chandra, MBBS, MD, PhD; Michael Stuart Bronze, MD; Barnett Gibbs, MD; Diane H Johnson, MD. “Naegleria Infection” eMedicine. 12 Feb 2009
- ↑ Drisdelle, Rosemary. "Naegleria fowleri - Deadly Amoeba suite101.com 11 Sep 2007
- ↑ 9.0 9.1 Alisky, Joseph Martin. "Survival of Naegleria fowleri Primary Amebic Meningocephalitis (PAM) could be Improved with an Intensive Multi-Route Chemo- and Biotherapeutic Regimen" "Medical Hypotheses" 2008 Volume 71, pgs. 969-971 26 June 2008
- ↑ Kim JH, D Kim, HJ Shin. "Contact-Independent Cell Death of Human Microglial Cells Due to Pathogenic Naegleria fowleri Trophozoites.” Korean Journal of Parasitology. 2008 Dec; 46(4):217-21. Epub 2008 Dec 20.
- ↑ Bennett, WM, JF Nespral, MW Rosson, KM McEvoy. "Use of Organs for Transplantation From a Donor with Primary Meningoencephalitis Due to Naegleria fowleri.” American Journal of Transplantation. 2008 Jun; 8(6): 1334-5. Epub 2008 Apr 29.