Hydrogen sulphide: Difference between revisions
imported>Milton Beychok m (Creation of a new article is in progress) |
mNo edit summary |
||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{ | {{Image|Hydrogen sulfide.png|right|150px|}} | ||
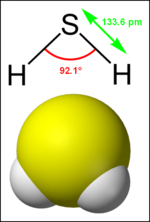
'''Hydrogen | '''Hydrogen sulphide''' (or ''hydrogen sulfide'') is a [[chemical compound]] with the [[chemical formula|formula]] [[Hydrogen|H]]<sub>2</sub>[[Sulphur|S]]. It is a colourless, highly toxic, flammable [[gas]] with a characteristic foul odour. | ||
Hydrogen | Hydrogen sulphide is present in very large amounts in raw [[natural gas]] and also occurs in large amounts during the [[Petroleum refining processes|refining]] of [[petroleum crude oil]]. In fact, the vast majority of the 66,000,000 [[metric ton]]s of elemental [[sulphur]] produced worldwide in 2006 was by-product sulphur from petroleum refining and [[Natural gas processing|natural gas processing plants]].<ref>[http://minerals.usgs.gov/minerals/pubs/commodity/sulphur/sulfumcs07.pdf Sulphur production report] by the [[United States Geological Survey]]</ref> It is also present in [[volcano|volcanic gases]] and some [[water]] well sources | ||
It also occurs in [[swamp]]s and [[sewer]]s as a result of the [[bacteria|bacterial]] breakdown of [[Organic chemistry|organic matter]] in the absence of [[oxygen]], which is known as [[anaerobic digestion]]. | It also occurs in [[swamp]]s and [[sewer]]s as a result of the [[bacteria|bacterial]] breakdown of [[Organic chemistry|organic matter]] in the absence of [[oxygen]], which is known as [[anaerobic digestion]]. | ||
{{TOC|left}} | |||
<BR/><BR/><BR/><BR/><BR/><BR/> | |||
{| border=0 width= | {| border=0 width=330 align=right style="clear: both;" | ||
| | | | ||
{| class = wikitable align=right style="font-size:90%;" | {| class = wikitable align=right style="font-size:90%;" | ||
| Line 15: | Line 16: | ||
! width=120px|Property!! Width=185px|Value</font><ref>{{cite book|author=Carl L. Yaws|title=Matheson Gas Data Handbook|edition=7th Edition|publisher=McGraw-Hill|date=June 25, 2001|id=ISBN 0-07-135851-4}}</ref><ref>[http://encyclopedia.airliquide.com/Encyclopedia.asp?GasID=59 Air Liquide Gas Encyclopedia]</ref> | ! width=120px|Property!! Width=185px|Value</font><ref>{{cite book|author=Carl L. Yaws|title=Matheson Gas Data Handbook|edition=7th Edition|publisher=McGraw-Hill|date=June 25, 2001|id=ISBN 0-07-135851-4}}</ref><ref>[http://encyclopedia.airliquide.com/Encyclopedia.asp?GasID=59 Air Liquide Gas Encyclopedia]</ref> | ||
|- align=left | |- align=left | ||
| '''Common name'''|| hydrogen | | '''Common name'''|| hydrogen sulphide | ||
|- align=left | |- align=left | ||
| '''Other names'''|| hydrogen sulphide, dihydrogen | | '''Other names'''|| hydrogen sulphide, dihydrogen sulphide, sulphur hydride, sewer gas | ||
|- align=left | |- align=left | ||
| '''IUPAC name''' || hydrogen | | '''IUPAC name''' || hydrogen sulphide | ||
|- align=left | |- align=left | ||
| '''CAS number'''|| 7783-06-04 | | '''CAS number'''|| 7783-06-04 | ||
| Line 35: | Line 36: | ||
| '''[[Heat of fusion]]''' ||69.74 kJ/kg at −85.47 °C and 1 atm | | '''[[Heat of fusion]]''' ||69.74 kJ/kg at −85.47 °C and 1 atm | ||
|- align=left | |- align=left | ||
| '''[[Heat of | | '''[[Heat of vaporisation]]'''||517.87 kJ/kg at −60.35 °C and 1 atm | ||
|- align=left | |- align=left | ||
| '''Liquid [[Density (chemistry)|density]]''' || 914.9 kg/m<sup>3</sup> at −60.35 °C and 1 atm | | '''Liquid [[Density (chemistry)|density]]''' || 914.9 kg/m<sup>3</sup> at −60.35 °C and 1 atm | ||
| Line 49: | Line 50: | ||
|} | |} | ||
Hydrogen | ==Chemical properties== | ||
Hydrogen sulphide is a highly [[toxic]] and flammable gas ([[flammability range]]: 4.0 to 46 volume % in air).<ref name=ChemInfo>[http://www.ccohs.ca/products/databases/samples/cheminfo.html CCOHS Chemical Name: Hydrogen sulphide] From the website of the [[Canadian Centre for Occupational Health and Safety]] (CCOHS)</ref> It is heavier than air and tends to accumulate at the bottom of poorly ventilated, confined spaces. | |||
Hydrogen | Hydrogen sulphide and oxygen burn to form [[sulphur dioxide]] (SO<sub>2</sub>) and water. In general, hydrogen sulphide acts as a [[reducing agent]]. | ||
At high temperature (about 1000 °[[Celsius (unit)|C]]) and with the use of a [[catalyst]], | At high temperature (about 1000 °[[Celsius (unit)|C]]) and with the use of a [[catalyst]], sulphur dioxide will react with hydrogen sulphide to form elemental sulphur and water (in the form of [[steam]]). This is commonly accomplished by the [[Claus process]], the primary method of converting hydrogen sulphide into elemental sulphur. | ||
Hydrogen | Hydrogen sulphide is readily soluble in water, amounting to 4.1 grams per litre of water at 20 °C which is equivalent to 0.41 weight %.<ref>[http://www.atsdr.cdc.gov/toxprofiles/tp114.pdf Toxicological Profile for Hydrogen Sulphide] July 2006, [[U.S. Department of Health and Human Services]], [[Agency for Toxic Substances and Disease Registry]] (ATSDR)</ref> When it is in solution in water, the H<sub>2</sub>S forms a weak acid (sometimes referred to as hydrosulphuric acid) containing hydrosulphide and sulphide [[ion]]s (HS<sup>−</sup> and S<sup>2−</sup>). The solubility is further enhanced in alkaline solutions due to increased ionisation. | ||
Hydrogen | Hydrogen sulphide reacts with metal ions to form metal [[sulphide]]s, which may be considered the [[salt]]s of hydrogen sulphide. Some [[Mineral deposit|minerals]] are sulphides. | ||
Hydrogen | Hydrogen sulphide reacts with alcohols to form [[thiol]]s. | ||
==Toxicity== | ==Toxicity== | ||
Hydrogen sulphide is considered a broad-spectrum poison, meaning that it can poison several different systems in the body, although the [[nervous system]] is most affected:<ref name=ChemInfo/><ref>[http://www.extension.iastate.edu/Publications/PM1963A.pdf Odor perception and physiological response]</ref><ref>{{cite book|author=Peter D. Bryson|title=Comprehensive Review In Toxicology For Emergency Clinicians|edition=Third Edition|publisher=Taylor and Francis|date=September 1996|pages=p. 368|id=ISBN 1-56032-612-3}}</ref> | |||
* 0.00047 [[parts-per notation|ppmv]] is the recognition threshold, the concentration at which 50% of humans can detect the characteristic foul odour of hydrogen sulphide. | |||
* 0.00047 [[parts-per notation|ppmv]] is the recognition threshold, the concentration at which 50% of humans can detect the characteristic foul | |||
* At 100 – 150 ppmv, the [[olfactory nerve]] is paralyzed after a few inhalations, and the sense of smell disappears, often together with awareness of danger. | * At 100 – 150 ppmv, the [[olfactory nerve]] is paralyzed after a few inhalations, and the sense of smell disappears, often together with awareness of danger. | ||
* At 200 ppmv, the central nervous system is depressed. | * At 200 ppmv, the central nervous system is depressed. | ||
| Line 72: | Line 73: | ||
==References== | ==References== | ||
{{reflist}} | {{reflist}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:00, 30 August 2024
Hydrogen sulphide (or hydrogen sulfide) is a chemical compound with the formula H2S. It is a colourless, highly toxic, flammable gas with a characteristic foul odour.
Hydrogen sulphide is present in very large amounts in raw natural gas and also occurs in large amounts during the refining of petroleum crude oil. In fact, the vast majority of the 66,000,000 metric tons of elemental sulphur produced worldwide in 2006 was by-product sulphur from petroleum refining and natural gas processing plants.[1] It is also present in volcanic gases and some water well sources
It also occurs in swamps and sewers as a result of the bacterial breakdown of organic matter in the absence of oxygen, which is known as anaerobic digestion.
|
Chemical properties
Hydrogen sulphide is a highly toxic and flammable gas (flammability range: 4.0 to 46 volume % in air).[4] It is heavier than air and tends to accumulate at the bottom of poorly ventilated, confined spaces.
Hydrogen sulphide and oxygen burn to form sulphur dioxide (SO2) and water. In general, hydrogen sulphide acts as a reducing agent.
At high temperature (about 1000 °C) and with the use of a catalyst, sulphur dioxide will react with hydrogen sulphide to form elemental sulphur and water (in the form of steam). This is commonly accomplished by the Claus process, the primary method of converting hydrogen sulphide into elemental sulphur.
Hydrogen sulphide is readily soluble in water, amounting to 4.1 grams per litre of water at 20 °C which is equivalent to 0.41 weight %.[5] When it is in solution in water, the H2S forms a weak acid (sometimes referred to as hydrosulphuric acid) containing hydrosulphide and sulphide ions (HS− and S2−). The solubility is further enhanced in alkaline solutions due to increased ionisation.
Hydrogen sulphide reacts with metal ions to form metal sulphides, which may be considered the salts of hydrogen sulphide. Some minerals are sulphides.
Hydrogen sulphide reacts with alcohols to form thiols.
Toxicity
Hydrogen sulphide is considered a broad-spectrum poison, meaning that it can poison several different systems in the body, although the nervous system is most affected:[4][6][7]
- 0.00047 ppmv is the recognition threshold, the concentration at which 50% of humans can detect the characteristic foul odour of hydrogen sulphide.
- At 100 – 150 ppmv, the olfactory nerve is paralyzed after a few inhalations, and the sense of smell disappears, often together with awareness of danger.
- At 200 ppmv, the central nervous system is depressed.
- At 500 ppmv, the cardiovascular system is depressed.
- At 1000 ppmv, the central nervous system is paralyzed and death occurs.
References
- ↑ Sulphur production report by the United States Geological Survey
- ↑ Carl L. Yaws (June 25, 2001). Matheson Gas Data Handbook, 7th Edition. McGraw-Hill. ISBN 0-07-135851-4.
- ↑ Air Liquide Gas Encyclopedia
- ↑ 4.0 4.1 CCOHS Chemical Name: Hydrogen sulphide From the website of the Canadian Centre for Occupational Health and Safety (CCOHS)
- ↑ Toxicological Profile for Hydrogen Sulphide July 2006, U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry (ATSDR)
- ↑ Odor perception and physiological response
- ↑ Peter D. Bryson (September 1996). Comprehensive Review In Toxicology For Emergency Clinicians, Third Edition. Taylor and Francis, p. 368. ISBN 1-56032-612-3.