Sildenafil: Difference between revisions
Pat Palmer (talk | contribs) m (spelling) |
mNo edit summary |
||
| (One intermediate revision by one other user not shown) | |||
| Line 14: | Line 14: | ||
|casnumber= | |casnumber= | ||
}} | }} | ||
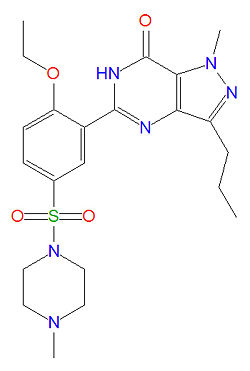
'''Sildenafil''', widely known as [[Viagra]]®, is a medication used to treat [[erectile dysfunction]].<ref name="pmid14581493">{{cite journal |author=Corbin JD, Francis SH |title=Molecular biology and pharmacology of PDE-5-inhibitor therapy for erectile dysfunction |journal=J. Androl. |volume=24 |issue=6 Suppl |pages=S38–41 |year=2003 |pmid=14581493 |doi= |url=http://www.andrologyjournal.org/cgi/pmidlookup?view=long&pmid=14581493 |issn=}}</ref> Low doses may also be used in prostate cancer | '''Sildenafil''', widely known as [[Viagra]]®, is a medication used to treat [[erectile dysfunction]].<ref name="pmid14581493">{{cite journal |author=Corbin JD, Francis SH |title=Molecular biology and pharmacology of PDE-5-inhibitor therapy for erectile dysfunction |journal=J. Androl. |volume=24 |issue=6 Suppl |pages=S38–41 |year=2003 |pmid=14581493 |doi= |url=http://www.andrologyjournal.org/cgi/pmidlookup?view=long&pmid=14581493 |issn=}}</ref> Low doses may also be used in prostate cancer radiation treatments to increase circulation in the groin area. Sildenafil is also marketed as Ravatio® as an oral treatment for pulmonary arterial hypertension. Both drugs are sold as the citrate salt of sildenafil. It was the first commercialized ''selective'' [[phosphodiesterase]] type 5 ([[PDE-5]]) inhibitor and was immediately popular both for treating erectile dysfunction and for recreational use. Sildenafil works by binding to phosphodiesterase type-5 enzymes, competing with the natural ligand [[cyclic guanine monophosphate]] (cGMP), which is structurally similar to sildenafil. [[Vardenafil]], a newer and more potent PDE-5 inhibitor, is nearly identical to sildenafil, while [[tadalafil]] is considerably different in structure. | ||
== Mechanism of action == | == Mechanism of action == | ||
| Line 35: | Line 35: | ||
== External links == | == External links == | ||
{{CZMed}} | {{CZMed}}[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 11:01, 18 October 2024
|
| |||||||
| sildenafil | |||||||
| |||||||
| Uses: | Erectile Dysfunction | ||||||
| Properties: | PDE-5 inhibitor | ||||||
| Hazards: | cardiovascular risks | ||||||
| |||||||
Sildenafil, widely known as Viagra®, is a medication used to treat erectile dysfunction.[1] Low doses may also be used in prostate cancer radiation treatments to increase circulation in the groin area. Sildenafil is also marketed as Ravatio® as an oral treatment for pulmonary arterial hypertension. Both drugs are sold as the citrate salt of sildenafil. It was the first commercialized selective phosphodiesterase type 5 (PDE-5) inhibitor and was immediately popular both for treating erectile dysfunction and for recreational use. Sildenafil works by binding to phosphodiesterase type-5 enzymes, competing with the natural ligand cyclic guanine monophosphate (cGMP), which is structurally similar to sildenafil. Vardenafil, a newer and more potent PDE-5 inhibitor, is nearly identical to sildenafil, while tadalafil is considerably different in structure.
Mechanism of action
By competitively binding to PDE-5 enzymes in smooth muscle and therefore inhibiting the binding of cGMP to PDE-5, the degradation of cGMP is reduced resulting in elevated levels of cGMP in the corpus cavernosum and its supply vessels. The elevated cGMP levels relax the smooth muscles, dilate the corporeal sinusoids and increase blood flow enabling an erection. cGMP levels are normally increased during stimulation by the release of nitric oxide in the corpus cavernosum. The nitric oxide activates guanylate cyclase, an enyme, which produces cGMP. Thus, sildenafil does not enhance the normal mechanism, namely increased synthesis of cGMP, but rather reduces its degradation.
Chemistry
The IUPAC name of sildenafil is 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo [4,3-d] pyrimidin-5-yl)-4-ethoxyphenyl] sulfonyl]-4-methylpiperazine and it has a molecular mass of 666.7 g/mol (as the citrate salt).
Drug interactions
Because sildenafil has vasodilator properties that result in decreased blood pressure, the combined use of sildenafil with other vasodilators, such as alpha-blockers, must be done cautiously. Patients with a history of heart attacks, strokes, arrythmia, hypertension, retinitis pigmentosa or currently on bosentan therapy should also be cautious.
Uses in medicine
Sildenafil can help treat erectile dysfunction according to a systematic review of randomized controlled trials.[2] In this review 83% of men who used sildenafil had at least one episode of intercourse as compared to 45% who received placebo.
Sildenafil can help pulmonary hypertension according to a randomized controlled trial.[3]
References
- ↑ Corbin JD, Francis SH (2003). "Molecular biology and pharmacology of PDE-5-inhibitor therapy for erectile dysfunction". J. Androl. 24 (6 Suppl): S38–41. PMID 14581493. [e]
- ↑ Fink HA, Mac Donald R, Rutks IR, Nelson DB, Wilt TJ (June 2002). "Sildenafil for male erectile dysfunction: a systematic review and meta-analysis". Arch. Intern. Med. 162 (12): 1349–60. PMID 12076233. [e]
- ↑ Galiè N, Ghofrani HA, Torbicki A, et al (November 2005). "Sildenafil citrate therapy for pulmonary arterial hypertension". N. Engl. J. Med. 353 (20): 2148–57. DOI:10.1056/NEJMoa050010. PMID 16291984. Research Blogging.
External links
The most up-to-date information about Sildenafil and other drugs can be found at the following sites.
- Sildenafil - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Sildenafil - Drug information for consumers from MedlinePlus (U.S. National Library of Medicine).
- Sildenafil - Detailed information from DrugBank.