Steroid: Difference between revisions
imported>David E. Volk (hormone receptors blurb) |
imported>David E. Volk mNo edit summary |
||
| Line 42: | Line 42: | ||
== hormone receptors and mechanism of action == | == hormone receptors and mechanism of action == | ||
Steroids primary act by increasing gene expression and therefore must enter the nuclei of the target cells to upregulated gene express of specific proteins. Because of this mode of action, steroid hormones take hours to have an effect because messenger RNA and then proteins must be synthesized. This is in contrast with other fast acting hormones, such as [[epinephrine]]. The formation of the messenger RNA can be suppressed by [[actinomycin D]]. Steroids first bind tightly, with dissociation constants in the nanomolar range, to their receptor protein. This complex is tranlocated into the cell nucleus and binds to specific operator sites in the DNA. All steroid receptors have a DNA-bind region, often called the DNA-binding domain (or DBD), which is rich is the positively-charged amino acids arginine and lysine, and also contains many cysteines. The presence of a "Cys-X-X-Cys" motif (X=any amino acid) suggests the presence of a metal-binding finger. | Steroids primary act by increasing gene expression and therefore must enter the nuclei of the target cells to upregulated gene express of specific proteins. Because of this mode of action, steroid hormones take hours to have an effect because messenger RNA and then proteins must be synthesized. This is in contrast with other fast acting hormones, such as [[epinephrine]]. The formation of the messenger RNA can be suppressed by [[actinomycin D]]. Steroids first bind tightly, with dissociation constants in the nanomolar range, to their receptor protein. This complex is tranlocated into the cell nucleus and binds to specific operator sites in the DNA. All steroid receptors have a DNA-bind region, often called the DNA-binding domain (or DBD), which is rich is the positively-charged amino acids arginine and lysine, and also contains many cysteines. The presence of a "Cys-X-X-Cys" motif (X=any amino acid) suggests the presence of a metal-binding finger. The receptors also have activatation factor domains at which additional cofactors must bind before the steroid:receptor complex can enter the nucleus or inititiate gene expression. Due to their complexity, hormone receptors are not well understood at this time and are the subject of intense research. However, no structures of steroid receptors in the unbound state have ever been determined to verify this. Several steroid:receptor complex structures have recently been determined. In addition to the DBD, all steroid hormone receptors have a hormone-binding region and the similarities in this region of the receptors suggests that they all had a common ancestor. The steroid receptor proteins are generally specific for each steroid class, binding tightly only to the target steroids, but weak binding can occur with other steroids for some of them. The [[thyroid-hormone receptor]] is similar in structure to the steroid receptors. | ||
Revision as of 15:30, 21 December 2007
Steroids, or steroid hormones, are powerful hormones with drastic effects, both good and bad, when artificially supplimented into living systems. They play a role in all stages of life from the embryo until death. Corticosteriods, or synthetic mimics such as prednisone, are used to treat inflamation related illnesses like asthma or rheumatoid arthritis, but they can have severe side effects. Athletes have often taken anobolic steroids to improve muscle growth and athletic performance. Glucocorticoids play a role in inflamation, and estrogens have been linked to cancer. Testosterone and estrogen influence sexual traits (maleness/femaleness). All steroid hormones are naturally synthesized from cholesterol (through pregnenolone) under the control of the anterior pituitary gland, which produces andrenocorticotropic hormone (ACTH, or corticotropin), a polypeptide that stimulates the conversion of cholesterol to pregnenolone. The five major classes of steroids are: progestagens, glucocorticoids, mineralcorticoids, androgens and estrogens. The steroid hormones activate gene expression by binding to enhancer proteins, called steroid receptors. Deficiency of the enzyme 21-hydroxylase is the most common steroid-related in-born metabolic disorder, but treatments are available for this condition.
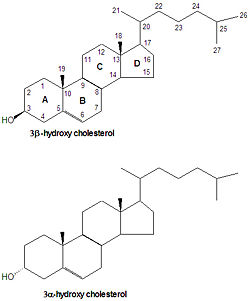
cholesterol
Cholesterol is the precursor from which all steroid hormones are synthesized. Because of this, all steroid numbering and nomenclature follow that of cholesterol. Some cholesterol derivatives have a proton added to the C-5 carbon. If the H-5 proton is -oriented (points down) then rings A and B are fused in a trans conformation, but in the orientation, the rings are fused in a cis conformation. All steroids with an H5 are in the 5 orientation, while bile salts derived from cholesterol have the opposite 5 orientation.
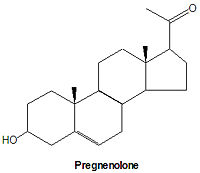
pregnenolone and progestagens
All steroid hormones have 21 or fewer carbons, although their precursor chemical, cholesterol, has 27 carbon atoms. Pregnenolone is the first steroid derived from cholesterol. It is synthesized through an intermediate, 20,22-dihydroxy-cholesterol, which is subsequently oxidized at C-20 to form a ketone with cleavage of carbons 22-27.
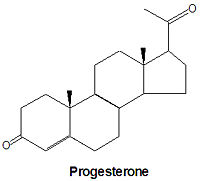
Progesterone, a progestagen which prepares the lining of the uterus for implantation of an ovum, is biosynthesized from prognenolone by oxidation of the 3-hydroxy group into a 3-keto group and by the isomerization of the 5 double bond into a 4 double bond. This hormone is also essential to maintain pregnancy. Progresterone is the precursor chemical in the biosynthesis of corticoids and androgens.
glucocorticoids and mineralcorticoids
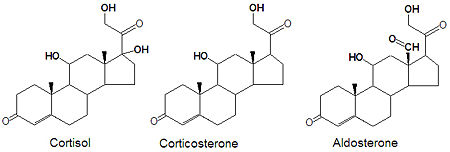
Glucocorticoids and mineralocorticoids (or mineralcorticoids) are naturally synthesized by the enzymatic oxidation of progesterone. Cortisol is produced when progesterone is hydroxylated at three positions, C-11, C-17 and C21. The oxidation of the C-17 carbon must occur before the hydroxylation at C-21 to synthesize cortisol, otherwise corticosterone is formed. Aldosterone, the major mineralcorticoid, is synthesized from corticosterone by oxidation of the C-18 methyl group to form an aldehyde.
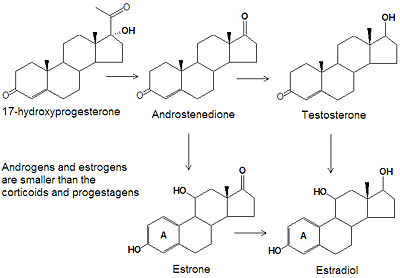
androgens and estrogens
While the progestagens, glucocorticoids and mineralcorticoids all have twenty one carbon atoms, the smaller androgens and estrogens have only nineteen or eighteen carbon atoms, respectively. The biosynthesis of both classes of steroids starts with the hydroxylation of progesterone at C-17 to produce 17<math\alpha</math>-hydroxyprogesterone. Cleavage of the C20-C21 side chain and oxidation of the C-17 hydroxyl group into a ketone produces androstenedione. Reduction of the C-17 keto group of androstenedione yields testosterone, one of the most abused anabolic steroids. The smallest class of steroids, the estrogens, are biosynthesized from the androgens by loss of the C-19 -methyl group, reduction of the C-3 ketone to a hydroxyl group, and the formation of an aromatic ring A. Estradiol is the major estrogen.
21-hydroxylase deficiency
Some people inherit defective forms of the enzyme 21-hydroxylase. Because 21-hydroxylase is a key enzyme in the production of the corticosteroids, defects in this enzyme lead to severe physiological effects. The enzyme is required for the production of cortisol, corticosterone, aldosterone and other steroids. Diminished levels of the glucocorticoids induces the anteterior pituitary gland to increase production of ACTH, which in turn incrases the levels of progesterone and 17hydroxy-progesterone. High levels of these two hormones increases the levels of androgens, which are derived from them. The pathogenesis can be stopped by the administration of glucocorticoids to complete the feed-back mechanism and halt overproduction of ACTH.
Aldosterone is the main mineralcorticoid and is produced in the adrenal glands. It plays a critical role in retaining sodium ion concentrations and thus hydration, so patients with 21-hydroxylase deficiency can suffer hypertension and dehydration, which can lead to shock or death. Increases in the levels of the androgens (testosterone, for example) resulting from the overproduction of progesterone and 17hydroxy-progesterone lead to the virilization ((masculinization) of those affected. In females, masculinization of the external genitalia is usually evident at birth. Males appear normal at birth but sexual precocity soon follows. Accellerated growth and early bone development leads to a shortened stature.
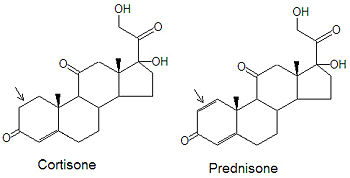
Cortisone and Prednisone
Although cortisone can be used to treat inflamation related diseases, like rheumatoid arthritis, it has severe side effects. The drug prednisone was developed as a cortisone mimic and its use to leads to milder side effect problems in the patient population. The only difference between the two compounds is the presence of an double bond between the C1 and C2 carbons of prednisone (note arrows in figure). Cortisone has a single C-C bond in this location.
hormone receptors and mechanism of action
Steroids primary act by increasing gene expression and therefore must enter the nuclei of the target cells to upregulated gene express of specific proteins. Because of this mode of action, steroid hormones take hours to have an effect because messenger RNA and then proteins must be synthesized. This is in contrast with other fast acting hormones, such as epinephrine. The formation of the messenger RNA can be suppressed by actinomycin D. Steroids first bind tightly, with dissociation constants in the nanomolar range, to their receptor protein. This complex is tranlocated into the cell nucleus and binds to specific operator sites in the DNA. All steroid receptors have a DNA-bind region, often called the DNA-binding domain (or DBD), which is rich is the positively-charged amino acids arginine and lysine, and also contains many cysteines. The presence of a "Cys-X-X-Cys" motif (X=any amino acid) suggests the presence of a metal-binding finger. The receptors also have activatation factor domains at which additional cofactors must bind before the steroid:receptor complex can enter the nucleus or inititiate gene expression. Due to their complexity, hormone receptors are not well understood at this time and are the subject of intense research. However, no structures of steroid receptors in the unbound state have ever been determined to verify this. Several steroid:receptor complex structures have recently been determined. In addition to the DBD, all steroid hormone receptors have a hormone-binding region and the similarities in this region of the receptors suggests that they all had a common ancestor. The steroid receptor proteins are generally specific for each steroid class, binding tightly only to the target steroids, but weak binding can occur with other steroids for some of them. The thyroid-hormone receptor is similar in structure to the steroid receptors.