Glucose: Difference between revisions
imported>David E. Volk No edit summary |
imported>David E. Volk m (typo) |
||
| Line 2: | Line 2: | ||
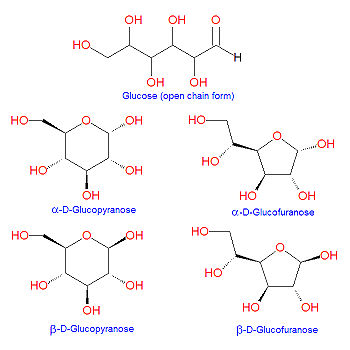
[[Image:Glucose structures.jpg|right|thumb|350px|{{#ifexist:Template:Glucose structures.jpg/credit|{{Glucose structures.jpg/credit}}<br/>|}}Different forms of glucose]] | [[Image:Glucose structures.jpg|right|thumb|350px|{{#ifexist:Template:Glucose structures.jpg/credit|{{Glucose structures.jpg/credit}}<br/>|}}Different forms of glucose]] | ||
'''Glucose''' is a [[hexose]] [[sugar]] molecule present in living organisms. It is the main form of energy in a cell, and the first step of [[glucolysis]] is the transformatin of glucose to [[glucose-6-phosphate]]. It is a component of the [[ | '''Glucose''' is a [[hexose]] [[sugar]] molecule present in living organisms. It is the main form of energy in a cell, and the first step of [[glucolysis]] is the transformatin of glucose to [[glucose-6-phosphate]]. It is a component of the [[disaccharide]]s [[sucrose]], [[galactose]] and [[maltose]], to name a few. Because it contains an [[aldehyde]] in the open chain structure, it is one of the [[aldose]] sugars. Glucose can cyclize to form five- and six-atom ring structures called [[glucofuranose]] and [[glucopyranose]], respectively, as shown in the illustration. In 1891, the German chemist [[Emil Fischer]] elucidated the structure of D-glucose. | ||
== Cyclization mechanism == | == Cyclization mechanism == | ||
Glucose can undergo [[intramolecular reaction]]s in which the aldehyde carbon at position C-1 is subject to nucleophilic attack by either the C-4 or C-5 hydroxyl groups to form cyclic [[hemiacetal]] structures called [[glucofuranose]] or [[glucopyranose]], respectively. Depending on the stereochemical orientation of the resulting C-1 hydroxyl group formed in this reaction, the products are labeled as alpha or beta (see illustration). | Glucose can undergo [[intramolecular reaction]]s in which the aldehyde carbon at position C-1 is subject to nucleophilic attack by either the C-4 or C-5 hydroxyl groups to form cyclic [[hemiacetal]] structures called [[glucofuranose]] or [[glucopyranose]], respectively. Depending on the stereochemical orientation of the resulting C-1 hydroxyl group formed in this reaction, the products are labeled as alpha or beta (see illustration). | ||
Revision as of 15:54, 6 February 2008
Glucose is a hexose sugar molecule present in living organisms. It is the main form of energy in a cell, and the first step of glucolysis is the transformatin of glucose to glucose-6-phosphate. It is a component of the disaccharides sucrose, galactose and maltose, to name a few. Because it contains an aldehyde in the open chain structure, it is one of the aldose sugars. Glucose can cyclize to form five- and six-atom ring structures called glucofuranose and glucopyranose, respectively, as shown in the illustration. In 1891, the German chemist Emil Fischer elucidated the structure of D-glucose.
Cyclization mechanism
Glucose can undergo intramolecular reactions in which the aldehyde carbon at position C-1 is subject to nucleophilic attack by either the C-4 or C-5 hydroxyl groups to form cyclic hemiacetal structures called glucofuranose or glucopyranose, respectively. Depending on the stereochemical orientation of the resulting C-1 hydroxyl group formed in this reaction, the products are labeled as alpha or beta (see illustration).