Lactam: Difference between revisions
imported>David E. Volk (cephalosporin structure added) |
imported>David E. Volk |
||
| Line 6: | Line 6: | ||
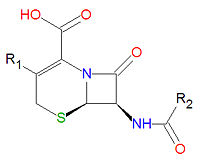
[[Image:Cephalosporin base.jpg|right|thumb|200px|<small>Base structure of all cephalosporins.</small>]] | [[Image:Cephalosporin base.jpg|right|thumb|200px|<small>Base structure of all cephalosporins.</small>]] | ||
The <math>\beta</math>-lactam forms the center structure of many antibiotic drugs, such as the [[cephalosporin]]s and the [[penicillin]]s, as shown above. | The <math>\beta</math>-lactam forms the center structure of many antibiotic drugs, such as the [[cephalosporin]]s and the [[penicillin]]s, as shown above. In the penicillins, the non-lactam ring is one atom smaller compared to the cephalosporins. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of the beta-lactam, which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins and penicillins are often made semi-synthetically, using a core structure obtained from a natural organism, such as a fungus, due to the difficulty and expense of synthesizing these lactams. | ||
Revision as of 14:42, 17 May 2008
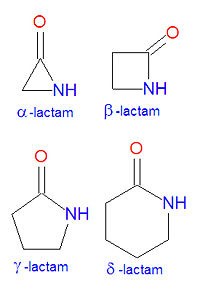
In chemistry, a lactam is a cyclic amide. The name is derived from two chemical terms, lactone, referring to a cyclic ketone, and amide, a compound containing a nitrogen atom next to a carbonyl group. Lactams are named according to the size of the cyclic ring in the lactam: -lactams, -lactams, -lactams and -lactams contain rings made of three, four, five or six atoms, respectively. -lactams are also called aziridinones. Many widely used antibiotic drugs, including thepenicillins and cephalosporins, owe their activity to the presence of a -lactam structure. The lactams may have substitutions added to the nitrogen atom or any of the non-carbonyl carbon atoms in the base structure.
Antibiotics
The -lactam forms the center structure of many antibiotic drugs, such as the cephalosporins and the penicillins, as shown above. In the penicillins, the non-lactam ring is one atom smaller compared to the cephalosporins. The activity of cephalosporins, penicillins, and some other antibiotics are due to the presence of the beta-lactam, which binds irreversibly, via acylation, to penicillin-binding proteins, thereby inhibiting the peptidogycan layer of bacterial cell wall synthesis. Cephalosporins and penicillins are often made semi-synthetically, using a core structure obtained from a natural organism, such as a fungus, due to the difficulty and expense of synthesizing these lactams.