Technetium: Difference between revisions
imported>Anthony.Sebastian (add data and citation) |
imported>Milton Beychok (Wiki link) |
||
| Line 17: | Line 17: | ||
'''Technetium''' is a [[Chemical elements|chemical element]], having the [[chemical symbol]] Tc. Its [[atomic number]] (the number of [[proton]]s) is 43. It has a [[Atomic mass#Standard atomic weights of the elements|standard atomic weight]] of 98.9062 g•mol<sup> −1</sup> and is a [[solid]] in its elemental form. | '''Technetium''' is a [[Chemical elements|chemical element]], having the [[chemical symbol]] Tc. Its [[atomic number]] (the number of [[proton]]s) is 43. It has a [[Atomic mass#Standard atomic weights of the elements|standard atomic weight]] of 98.9062 g•mol<sup> −1</sup> and is a [[solid]] in its elemental form. | ||
Technetium is considered to be a member of the "Transition metal" class of elements.<ref>'''Note:''' Technitium is also sometimes referred to being a member of a ''Synthetic'' or ''Quasi-synthetic'' class of elements.</ref> At a [[pressure]] of 101.325 k[[Pascal (unit)|Pa]], it has a [[boiling point]] of 4,265 °[[Celsius (unit)|C]] and a melting point of 2,157 °C. | Technetium is considered to be a member of the "Transition metal" class of elements.<ref>'''Note:''' Technitium is also sometimes referred to being a member of a ''Synthetic'' or ''Quasi-synthetic'' class of elements.</ref> At a [[pressure]] of 101.325 k[[Pascal (unit)|Pa]], it has a [[boiling point]] of 4,265 °[[Celsius (unit)|C]] and a [[melting point]] of 2,157 °C. | ||
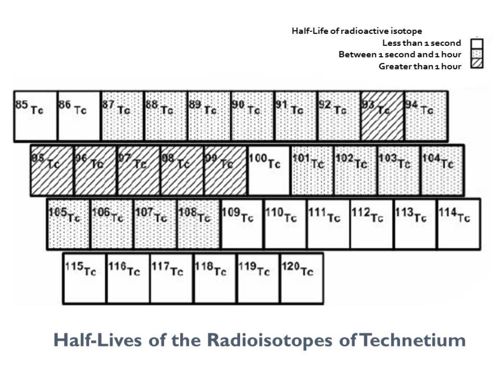
All the isotopes of technetium are radioactive; 98 is the atomic mass of technetium's longest-lived isotope, <sup>98</sup>Tc (4.12x10<sup>6</sup>y).<ref>[http://periodictable.com/Isotopes/043.98/index.html Technetium Isotope data].</ref> Technetium is the lightest chemical element lacking a stable isotope. | All the isotopes of technetium are radioactive; 98 is the atomic mass of technetium's longest-lived isotope, <sup>98</sup>Tc (4.12x10<sup>6</sup>y).<ref>[http://periodictable.com/Isotopes/043.98/index.html Technetium Isotope data].</ref> Technetium is the lightest chemical element lacking a stable isotope. | ||
Revision as of 20:05, 24 April 2011
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Technetium is a chemical element, having the chemical symbol Tc. Its atomic number (the number of protons) is 43. It has a standard atomic weight of 98.9062 g•mol −1 and is a solid in its elemental form.
Technetium is considered to be a member of the "Transition metal" class of elements.[1] At a pressure of 101.325 kPa, it has a boiling point of 4,265 °C and a melting point of 2,157 °C.
All the isotopes of technetium are radioactive; 98 is the atomic mass of technetium's longest-lived isotope, 98Tc (4.12x106y).[2] Technetium is the lightest chemical element lacking a stable isotope.
Only very small amounts of technetium are found in nature. Practically all technetium is produced synthetically as a by-product of the fission of uranium-235 in nuclear reactors and it is extracted from the spent reactor fuel rods.[3]
Technetium radioisotope half-lives
References
- ↑ Note: Technitium is also sometimes referred to being a member of a Synthetic or Quasi-synthetic class of elements.
- ↑ Technetium Isotope data.
- ↑ John Elmsley (2001). Nature's Building Blocks: An A-Z Guide to the Elements, 1st Edition. Oxford University Press. ISBN 0-19-850341-5.