Biomass: Difference between revisions
imported>Milton Beychok m (Added another photo. Also bolded the captions of most of the images.) |
imported>Milton Beychok m (→Fischer-Tropsch synthesis of liquid hydrocarbons: Fixed a spelling typo) |
||
| Line 84: | Line 84: | ||
:<math>\mathrm{(2n+1)\,H_2} + \mathrm{n\,CO} \rightarrow \mathrm{C_nH_{2n+2}} + \mathrm{n\,H_2O}</math> | :<math>\mathrm{(2n+1)\,H_2} + \mathrm{n\,CO} \rightarrow \mathrm{C_nH_{2n+2}} + \mathrm{n\,H_2O}</math> | ||
where n is an integer. For example, if n = 5, the equation represents the formation of [[pentane]] (C<sub>5</sub>H<sub>12</sub>) which is a liquid hydrocarbon that is a component of [[gasoline]] (petrol). As another example, if n = 12, the equation represents the formation of [[ | where n is an integer. For example, if n = 5, the equation represents the formation of [[pentane]] (C<sub>5</sub>H<sub>12</sub>) which is a liquid hydrocarbon that is a component of [[gasoline]] (petrol). As another example, if n = 12, the equation represents the formation of [[dodecane]] (C<sub>12</sub>H<sub>26</sub>) which is a liquid hydrocarbon component of diesel fuel. | ||
Lesser amounts of [[alkene]]s (C<sub>n</sub>H<sub>2n</sub>) and [[alcohol]]s (C<sub>n</sub>H<sub>2n+1</sub>OH) are formed in accordance with these equations:<ref name=knowl/> | Lesser amounts of [[alkene]]s (C<sub>n</sub>H<sub>2n</sub>) and [[alcohol]]s (C<sub>n</sub>H<sub>2n+1</sub>OH) are formed in accordance with these equations:<ref name=knowl/> | ||
Revision as of 23:22, 13 December 2011
Biomass, a source of renewable energy, is biological material such as wood, wood waste, municipal solid waste, straw, sugar cane, algae, and many other byproducts derived from agricultural and forestry production as well as other sources. Since biomass derives from plants generated by solar energy in the photosynthesis process, it can also be defined as the biological material on Earth that has stored solar energy in the chemical bonds of the organic material.
The fossil fuels (coal, petroleum and natural gas) are currently thought to have been formed from prehistoric, ancient biomass buried deeply underground over millions of years of geological time. Therefore, they are not considered to be renewable sources of energy
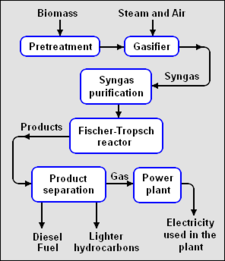
Production of fuels and other products from biomass
Biomass fuel for electric power production
The direct combustion of biomass for producing heat and electric power provides a ready disposal mechanism for municipal, agricultural, and industrial organic wastes. In 2009, about 11,350 megawatts (MW) of electric power, amounting to 1.1% of the summertime electrical supply in the United States was generated by burning biomass that included: wood, wood waste, municipal solid waste (MSW), landfill gas, and agricultural byproducts and waste.[1]
The New Hope Power Partnership in Florida is the largest biomass power plant in North America. It generates 140 MW of power using uses sugar cane fiber (bagasse) and recycled wood as fuel.[2] It has been in operation for more than 10 years.
Production of liquid transportation fuels
There are several processes available for converting the chemical energy contained in biomass into liquid automotive transport fuels such as biodiesel and ethanol.[3]
Ethanol fuel:
Ethanol fuel is ethyl alcohol (C2H5OH) and it is most often used as an automotive motor fuel, mainly as an additive for gasoline. Ethanol can be produced by fermentation of sugar cane, bagasse, sugar beets, barley, potatoes, corn and many other grains as well as many agricultural byproducts and wastes.
The worldwide production of ethanol for automotive fuel in 2007 was 52,000,000,000 litres (13,700,000,000 gallons). From 2007 to 2008, the share of ethanol in global gasoline use increased from 3.7% to 5.4%.[4] In 2009, worldwide ethanol fuel production reached 73,900,000,000 litres (19,500,000,000 gallons) and was expected to reach 85,900,000,000 litres (22,700,000,000 gallons) in 2010.[5]
Ethanol fuel is widely used in Brazil and in the United States, and together both countries were responsible for about 86 percent of the world's ethanol fuel production in 2009.[6]
Biodiesel fuel:
Biodiesel refers to a diesel fuel produced by chemically reacting lipids such as vegetable oils or animal fats with an alcohol such as methyl alcohol (CH3OH). The resulting biodiesel consists of esters of long-chain fatty acids. The process is known as "transesterification" and it may be carried out by several methods: the common batch process, supercritical processes and ultrasonic methods.
In 2009, the worldwide production of biodiesel was 17,900,000,000 litres (4,730,000,000 gallons). The three countries with the largest annual biodiesel production were Germany (16%), France (12%) and the United States (11%).[7]
|
Biomass gasification to produce syngas
Gasification is a process that reacts carbon-containing materials (such as coal or biomass) at high temperatures with reagent gases (namely, steam, pure oxygen and/or air) to produce a mixture of carbon monoxide (CO) and hydrogen (H2) commonly called syngas (a contraction of synthesis gas). It has been in use since the early 1800s when coal and peat were gasified to produce what was then called town gas for lighting and cooking ... later to be replace by electricity and natural gas. As of 2010, 412 modern industrial-scale gasifiers were in operation in 29 countries worldwide (see adjacent table).[9]
The syngas may be directly burned as a fuel, converted into methyl alcohol or hydrogen, subjected to methanation to produce synthetic natural gas (SNG),[10] or converted into synthetic liquid hydrocarbons via the Fischer-Tropsch process.
The vessel in which the gasification process takes place is called a gasifier. There are various types of gasifiers in current use,[9][11] including:
- Counter-current fixed bed gasifier: This gasifier is a vertical, cylindrical vessel containing a bed of carbon-containing material (e.g., coal or biomass) through which steam, oxygen and/or air flow upward. Although commonly referred to as a fixed bed gasifier, the crushed coal or biomass is fed into the gasifier through a lock hopper mounted on top of the gasifier and the carbonaceous material slowly moves downward as it is converted into syngas, which is removed from the upper portion of the gasifier, and ash or slag removed from the bottom of the gasifier.[12][13][14]
- Co-current fixed bed gasifier: This gasifier is also a vertical cylindrical vessel, which is quite similar to the counter-current gasifier type except that the steam, oxygen and/or air enter at the top of the bed, flow downward co-current with the slowly moving bed of carbonaceous material, and the product syngas is removed below the bed. [12][15][16]
- Fluidized bed gasifier: This gasifier is again a vertical, cylindrical vessel. It differs from the fixed bed reactors in that the feedstock biomass is very finely crushed before it enters the gasifier and the reaction bed of ground biomass is fluidized by the reagent gases (steam, oxygen and/or air) flowing upward through the bed. The fluidization of the reaction bed increases the height of the bed (as compared to the fixed bed gasifiers) and the finely crushed biomass results in much more biomass surface area being exposed to the reagent gases. The product syngas contains fine particles of crushed biomass which are removed by routing the syngas through a cyclone to recover the fines and recycle them back into the reaction bed.[12][17]
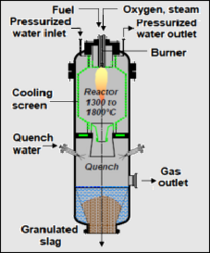
- Entrained flow gasifier: This gasifier, like the other three discussed above, is also a vertical, cylindrical vessel. The feedstock biomass is finely crushed and flows downward co-currently with pure oxygen. Air is seldom used as a reagent gas in an entrained gasifier. The gasification reactions take place in a dense cloud of fine biomass particles. The syngas exits from the bottom section of the gasifier and is routed through a cyclone (and perhaps a water scrubber) for removal of the fines. Entrained gasifiers operate at temperatures of about 1000 to 1800 °C and at pressures of 30 to 70 bar which is a much higher pressure than is the case for the other types of gasifiers. The adjacent Fig. 1 depicts an entrained flow gasifier of the type discussed here.[9][12][17][18]
Gasification chemistry
It is difficult to state exactly what chemical reactions occur during gasification. However, most researchers have reached agreement on the primary reactions and the syngas composition ranges shown just below:[9][12]
Fischer-Tropsch synthesis of liquid hydrocarbons
Fischer-Tropsch synthesis is a catalytic chemical process developed in the 1920s to convert a mixture of carbon monoxide and hydrogen (i.e., syngas), into hydrocarbon molecular chains of varying lengths.
The process was invented by Franz Fischer and Hans Tropsch working in Germany's Kaiser Wilhelm Institute (today the Max Planck Institute) in the 1920's. It was first commercialized in Germany in 1936 and it was used extensively by Germany during World War II to produce synthetic fuels. Later, facing isolation and embargoes during the apartheid era (1948 - 1990), South Africa turned to coal gasification and the Fischer-Tropsch process to supply significant quantities of its hydrocarbon fuel needs. Since that time, the process has been extensively improved and refined.[19][20]
The process is relatively simple: syngas is fed at high temperatures through catalysts (usually the transition metals cobalt or iron) which facilitate the hydrocarbon formation. When paired with syngas from the gasification of fossil fuels or biomass, the Fischer-Tropsch process is a method of converting any available carbon source into hydrocarbon liquids consisting of molecules having from 5 to 20 carbon atoms in accordance with the following equation for the formation of alkanes (CnH2n+2), which are the primary hydrocarbons formed:[19][21]
where n is an integer. For example, if n = 5, the equation represents the formation of pentane (C5H12) which is a liquid hydrocarbon that is a component of gasoline (petrol). As another example, if n = 12, the equation represents the formation of dodecane (C12H26) which is a liquid hydrocarbon component of diesel fuel.
Lesser amounts of alkenes (CnH2n) and alcohols (CnH2n+1OH) are formed in accordance with these equations:[21]
There are a good many different reactor designs for the Fischer-Tropsch process as well as different operating conditions. There are two general process temperature ranges:[21][22]
- Low temperature (200 to 260 °C): Designs using this temperature are referred to LTFT (a contraction of "low temperature Fischer-Tropsch") and generally use a cobalt catalyst.
- High temperature (300 to 350 °C): Designs using this temperature are referred to HTFT (a contraction of "high temperature Fischer-Tropsch")and generally use an iron catalyst.
The LTFT designs produce predominantly hydrocarbons in the boiling range of diesel fuel and waxes. Fixed-bed reactors are considered to be the "conventional" technology for LTFT designs and slurry phase reactors are considered to be "advanced" technology.
The HTFT designs produce predominantly gaseous alkene hydrocarbons having 2 to 4 carbon atoms and liquid hydrocarbons in the boiling range of gasoline. Circulating fluidized bed reactors are considered to be the "conventional" technology for HTFT designs and fixed fluidized bed reactors are considered to be "advanced" technology.
References
- ↑ U.S. Electric Net Summer Capacity U.S. Energy Information Administration (EIA), part of the U.S. Department of Energy (DOE)
- ↑ Agriculture & Renewable Energy: The Partnership for a New Frontier Florida Power Service Commission (FPSC) Workshop, July 26, 2007.
- ↑ ABC's of Biofuels From the website of the Office of Energy Efficiency and Renewable Energy (EERE) in the U.S. Department of Energy (DOE).
- ↑ Assessing Biofuels (2009) From the website of the United Nations Environment Programme (UNEP)
- ↑ Global ethanol production to reach 85.9 billion litres (22.7 billion gallons) in 2010 March 22, 2010. From the website of the Renewable Fuels Association (RFA).
- ↑ Ethanol Industry Statistics 2009 World Fuel Ethanol Production from the website of the Renewable Fuels Association.
- ↑ Production of biofuels in the world in 2009 August 30, 2010. From the website of the Biofuels Platform published in Switzerland.
- ↑ Worldwide Gasification Database National Energy Technology Laboratory (NETL), U.S. Department of Energy (DOE)
- ↑ 9.0 9.1 9.2 9.3 DOE Gasification Program Overview Jenny B. Tennant (December 2010, National Energy Technology Laboratory (NETL), U.S. Department of Energy. An excellent overview of the worldwide gasification technology.
- ↑ M.R. Beychok (May 1975), "Process and environmental technology for producing SNG and liquid fuels", U.S. EPA report EPA-660/2-75-011.
- ↑ Gasifiers in detail: Types of gasifiers National Energy Technology Laboratory ((NETL), U.S. Department of Energy (DOE)
- ↑ 12.0 12.1 12.2 12.3 12.4 Generic gasifier modelling: Evaluating model by gasifier type J.P. Visagie (October 2008), Master's dissertation, University of Pretoria, South Africa
- ↑ M.R. Beychok (March 1974). "Coal gasification for clean energy". Energy Pipelines and Systems.
- ↑ M.R. Beychok (September 1974), "Coal gasification and the Phenosolvan process", American Chemical Society 168th National Meeting, Atlantic City, New Jersey
- ↑ M.J. Groeneveld and W.P.M. Van Swaaij, "The Design of Co-Current Moving-Bed Gasifiers Fueled by Biomass" published in this book: Jerry Latham Jones and Shirley B. Radding (Editors) (1980). Thermal conversion of solid wastes and biomass: based on a symposium, Volume 130 of ACS symposium series. American Chemical Society. ISBN 0-8412-0565-5.
- ↑ Gasification and solid waste -- Potential and application of co-current moving bed gasifiers M.J. Groeneveld and W.P.M. Van Swaaij, Twente University of Technology, The Netherlands.
- ↑ 17.0 17.1 Fischer-Tropsch Fuels from Coal and Biomass Thomas G. Kreutz et al (October 2008), Princeton University, presented at 25th Annual International Pittsburgh Coal Conference, Pittsburgh, Pennsylvania, September 29 to October 2, 2008.
- ↑ Entrained Flow Gasification of Biomass A. van der Drift et al (April 2004), Energy research Centre of the Netherlands (ECN), Report ECN-C--04-039.
- ↑ 19.0 19.1 Fischer-Tropsch (FT) Synthesis] From the website of the National Energy Technology Laboratory (NETL) in the U.S. Department of Energy.
- ↑ Hans Schulz (October 4, 1999). "Short history and present trends of Fischer–Tropsch synthesis". Applied Catalysis A: General 186 (1 - 2): pp. 3 - 12.
- ↑ 21.0 21.1 21.2 The Fischer-Tropsch (FT) Process From the Knowl website
- ↑ Fischer-Tropsch Synthesis Burtron H. Davis (2010) in AccessScience, McGraw-Hill Companies.
- Pages using ISBN magic links
- CZ Live
- Engineering Workgroup
- Chemistry Workgroup
- Biology Workgroup
- Chemical Engineering Subgroup
- Environmental Engineering Subgroup
- Articles written in American English
- All Content
- Engineering Content
- Chemistry Content
- Biology Content
- Chemical Engineering tag
- Environmental Engineering tag