Adipocyte: Difference between revisions
imported>Anthony.Sebastian (adding content, tweaking language) |
imported>Anthony.Sebastian (add citation note) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
The '''adipocyte''', a cell type located in adipose tissue, stores excess fat and makes it available on demand to the body system for use as energy. Adipocytes can also secrete signaling messengers, called ''adipokines''. Adipose tissue — which consists of other cell types besides adipocytes — operates as an endocrine organ, secreting more than 50 known adipokines. There are two different types of adipose tissue, white and brown. ''White adipose tissue'' (WAT) is the fat storage tissue and ''brown adipose tissue'' (BAT) produces heat. BAT is sometimes popularly called ‘baby fat’ as it is present in babies but converts to WAT in adulthood. This conversion is performed by mitochondrial uncoupling protein 1 (UCP1), which is activated by the diet, in the [[mitochondria]]. Mice that are genetically made to express UCP1 in their WAT become obesity resistant, and this has sparked interest in a possible treatment for obesity, by identifying what causes the UCP1 to stop working and so convert BAT to WAT, and then finding a way to stop that so that excess energy is released as heat rather than stored in WAT resulting in obesity. | The '''adipocyte''', a cell type located in adipose tissue, stores excess fat and makes it available on demand to the body system for use as energy. Adipocytes can also secrete signaling messengers, called ''adipokines''. Adipose tissue — which consists of other cell types besides adipocytes — operates as an endocrine organ, secreting more than 50 known adipokines.<ref>'''''Note:''''' Adipokines influence such processes as inflammation, control of appetite and energy balance, sensitivity to insulin, the metabolism of fats, and the growth of new blood vessels.</ref> There are two different types of adipose tissue, white and brown. ''White adipose tissue'' (WAT) is the fat storage tissue and ''brown adipose tissue'' (BAT) produces heat. BAT is sometimes popularly called ‘baby fat’ as it is present in babies but converts to WAT in adulthood. This conversion is performed by mitochondrial uncoupling protein 1 (UCP1), which is activated by the diet, in the [[mitochondria]]. Mice that are genetically made to express UCP1 in their WAT become obesity resistant, and this has sparked interest in a possible treatment for obesity, by identifying what causes the UCP1 to stop working and so convert BAT to WAT, and then finding a way to stop that so that excess energy is released as heat rather than stored in WAT resulting in obesity. | ||
Fat cells were first considered to be able to sense energy demands and signal to decrease food intake in the 1950’s. However it was not confirmed until 1973, when Coleman, using parabiosis, provided evidence that a circulating factor existed that signalled information about the body's energy requirements. This idea gained little attention until Siiteri in 1987 examined the role of obesity in cancers of the reproductive tract and found that adipocytes secreted the hormone [[oestrogen]] which is implicated in breast and endometrial cancers. Oestrogen is produced by the aromatase enzyme which is present in many tissues including adipose tissue. The production of oestrogen is accelerated in people that are obese, as more adipose tissue means more oestrogens and this is correlated with a higher incidence of cancers in the reproductive tract in obese individuals. Another prospect which supported the adipocyte as a regulator of energy stores came when adipsin, a serine protease, was also discovered to be secreted by adipocytes, and has since been found to be deficient in some animal models of obesity. | Fat cells were first considered to be able to sense energy demands and signal to decrease food intake in the 1950’s. However it was not confirmed until 1973, when Coleman, using parabiosis, provided evidence that a circulating factor existed that signalled information about the body's energy requirements. This idea gained little attention until Siiteri in 1987 examined the role of obesity in cancers of the reproductive tract and found that adipocytes secreted the hormone [[oestrogen]] which is implicated in breast and endometrial cancers. Oestrogen is produced by the aromatase enzyme which is present in many tissues including adipose tissue. The production of oestrogen is accelerated in people that are obese, as more adipose tissue means more oestrogens and this is correlated with a higher incidence of cancers in the reproductive tract in obese individuals. Another prospect which supported the adipocyte as a regulator of energy stores came when adipsin, a serine protease, was also discovered to be secreted by adipocytes, and has since been found to be deficient in some animal models of obesity. | ||
Revision as of 16:45, 26 November 2007
The adipocyte, a cell type located in adipose tissue, stores excess fat and makes it available on demand to the body system for use as energy. Adipocytes can also secrete signaling messengers, called adipokines. Adipose tissue — which consists of other cell types besides adipocytes — operates as an endocrine organ, secreting more than 50 known adipokines.[1] There are two different types of adipose tissue, white and brown. White adipose tissue (WAT) is the fat storage tissue and brown adipose tissue (BAT) produces heat. BAT is sometimes popularly called ‘baby fat’ as it is present in babies but converts to WAT in adulthood. This conversion is performed by mitochondrial uncoupling protein 1 (UCP1), which is activated by the diet, in the mitochondria. Mice that are genetically made to express UCP1 in their WAT become obesity resistant, and this has sparked interest in a possible treatment for obesity, by identifying what causes the UCP1 to stop working and so convert BAT to WAT, and then finding a way to stop that so that excess energy is released as heat rather than stored in WAT resulting in obesity.
Fat cells were first considered to be able to sense energy demands and signal to decrease food intake in the 1950’s. However it was not confirmed until 1973, when Coleman, using parabiosis, provided evidence that a circulating factor existed that signalled information about the body's energy requirements. This idea gained little attention until Siiteri in 1987 examined the role of obesity in cancers of the reproductive tract and found that adipocytes secreted the hormone oestrogen which is implicated in breast and endometrial cancers. Oestrogen is produced by the aromatase enzyme which is present in many tissues including adipose tissue. The production of oestrogen is accelerated in people that are obese, as more adipose tissue means more oestrogens and this is correlated with a higher incidence of cancers in the reproductive tract in obese individuals. Another prospect which supported the adipocyte as a regulator of energy stores came when adipsin, a serine protease, was also discovered to be secreted by adipocytes, and has since been found to be deficient in some animal models of obesity.
Obesity brings with it associated complications including cardiovascular problems, some cancers and type 2 diabetes mellitus. As cases of obesity are increasing in adults, so are cases of diabetes but perhaps more alarmingly is the increase in obesity related diabetes in children. The reason for the strong relationship between obesity and type 2 diabetes mellitus (T2DM) is still largely unknown and so there is currently a lot of research into how obesity can lead to T2DM.
Resistin
In 2001, an article in the Washington Post read, ‘Hormone may be key to diabetes’ (Washington Post, Jan 18th, 2001). The hormone was resistin, secreted from adipocytes of white adipose tissue, and it had been found to be involved in the emergence of T2DM. Resistin blood serum levels were significantly increased in cases of obesity and it was found to interfere with the actions of insulin and with glucose tolerance, causing insulin resistance in mice. This was also supported by research showing that deliberately induced diabetes, caused by provision of a high fat diet, caused obesity and elevated resistin levels in mice. These effects could be reversed by administration of a resistin antibody (Steppan et al, 2001). This discovery sparked excitement, as resistin appeared to be a candidate for the missing link between obesity and T2DM. However studies in humans provided disappointing, showing no significant differences between blood serum resistin levels in lean and obese people and no differences between serum resistin levels in healthy and diabetic people. These conclusions imply that resistin does not play a role in obesity related diabetes and does not appear to be governed by adiposity. The differences between humans and mice may be due to differences in energy metabolism and in genetic differences between the species with the mouse resistin gene not identical to the human gene, indeed only showing 59% homology (Lee et al, 2003).
More recent studies have found supernormal plasma concentrations of resistin in humans (South Koreans) with T2DM (Youn et al., 2004), though the studies yielded no insight into the role of the higher resistin levels. They were not related to gender or degree of obesity or insulin resistance (Youn et al., 2004). Saudi Arabians with T2DM also have increased plasma resistin levels when assessed for the presence of the so-called metabolic syndrome, an array of risk factors for cardiovascular disease (e.g., obesity in and around the abdomen; increased blood levels of those lipids that predispose to atherosclerosis; impaired ability to use insulin or blood sugar; indications of inflammation in the body) (Al-Daghri et al., 2007). That association of high resistin levels and the metabolic syndrome has been confirmed by other investigators (Norata et al., 2007). In a Turkish study group, investigators found that one specific form of the resistin gene (polymorphism) associated with insulin resistance and obesity in patients with T2DM (Duman et al., 2007). Taken together, those findings do link resistin and T2DM, but shed no light on the functional role of resistin in humans. Because in addition to adipocytes, cells involved in immunity and inflammation produce resistin, the link between resistin and T2DM might relate to inflammatory reactions in T2DM, an area needing further investigation (Lago et al., 2007; Anderson et al., 2007).
Visfatin
Another adipokine implicated in the obesity-diabetes saga is the molecule visfatin. Visfatin is secreted by visceral fat and has glucose-lowering effects similar to those of insulin. Visfatin and insulin stimulate muscle and adipose cells to take up glucose and restrain glucose release from hepatocytes. However, visfatin is unlike insulin in that its levels are constant regardless of food intake whereas insulin levels change depending on the feeding state. Visfatin also has significantly lower intracellular levels than insulin but interestingly it acts on the insulin receptor but not competitively. This may mean that visfatin is a mimetic of insulin, but its levels are low enough so that it does not interfere with the actions of insulin.
So fat cells are no longer considered to be just a fat store, but are dynamic cells that work to maintain the body's energy homeostasis. Many other molecules have been identified that are secreted by adipocytes, some those not involved in energy homeostasis eg. the cytokines.
Leptin
Probably the biggest breakthrough for the study of appetite regulation came in 1994 when the molecular geneticist Jeffrey Friedman discovered the adiposity signal leptin. Using the ob/ob mice which were thought to lack a satiety signal, Friedman and colleagues found that 'ob' codes for a gene which they called leptin, from the Greek ‘leptos’ meaning thin. Mice deficient in this gene are morbidly obese and this obesity can be reversed by giving the mice leptin. The leptin receptor was found in 1995 and is a member of the cytokine receptor family.
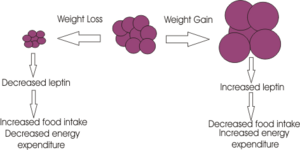
Leptin is a signalling molecule released from adipocyte cells to signal regarding adiposity levels. It is secreted into the blood, secretion proportional to body fat, where it travels to the brain causing a decrease in appetite through acting on specific neurones in the brain.
Unsurprisingly there was huge media interest in leptin as a possible treatment for obesity, and the biotechnology company Amgen paid $20 million to license leptin. However the results of clinical trials on obese people were disappointing, with very few of the participants losing weight. It appeared that most people were not obese because of a deficiency in leptin, but were just unable to respond to it; they had more fat cells and so more circulating leptin and as a result reduced sensitivity of the leptin receptor. The failure of the trial, however, overshadowed the fact that about 30% of the subjects lost weight and so may have low circulating levels of leptin. Potentially these people might benefit from leptin therapy. The task now though is to determine those people that have a lower circulating level of leptin.
The use of leptin as a drug has proved valuable in the disease lipodystrophy and in people who have a defective leptin gene and so are constantly hungry.
Leptin inhibits food intake by acting in the appetite control centres of the brain. Leptin receptor mRNA is found primarily in the hypothalamic arcuate nucleus, ventromedial nuclei and dorsomedial hypothalamic nuclei, regions that are known to be involved in appetite control.
The weight loss caused by leptin seems to arise because of two effects;
1. Inhibiting appetite through its actions on the appetite-stimulating neuropeptide Y neurones and the appetite-inhibiting proopiomelanocortin (POMC) neurons, located in the hypothalamic arcuate nucleus. Leptin inhibits the NPY neurons,causing a decrease in the release of the inhibitory neurotransmitter GABA (which is synthesised by the NPY neurons). This "disinhibits" the POMC neurons which increase their firing rate leading to the release of alpha MSH – a product of POMC wich is a potent inhibitor of appetite. Leptin also acts directly on the POMC neurons.
2. Leptin also increases energy expenditure by increasing body temperature and oxygen consumption
As yet it is still unknown as to how exactly leptin exerts its inhibitory effects on feeding behaviour, clearly if this were known then the potential to harness its effects for the treatment of eating disorders would be an enticing prospect. It acts on the JAK/STAT pathway, effecting gene transcription and other cellular signalling pathways but a definitive mode of action has not been found; leptin's mode of action probably involves several different signalling pathways.
Leptin is synthesised in many other tissues including the stomach, ovary, placenta and liver and its receptors are also located in a diverse range of tissues. It plays a role in many other physiological functions such as reproduction, where levels of leptin appear to dictate the commencement of menstruation and in foetal development where there is a surge in leptin levels in the first week of life, which does not correspond to a decrease in food intake but is thought to be a developmental signal. During development, mice deficient in leptin have disruptions in the arcuate nucleus neural projections, but not other hypothalamic projections, and these effects can be reversed upon leptin treatment.
Leptin’s primary function is still under consideration as it also plays a role in many other physiological processes. For example it can be thought of as a signal informing the body when it has sufficient fat to accommodate an ‘expensive’ physiological function like reproduction. Leptin therefore appears to protect the body against starvation by only allowing energy dense processes to occur when the body is sufficiently ready. Its primary role is complex, is it a satiety signal to prevent overeating or an evolutionarily efficient signal protecting against starvation?
References
Flier J et al. (1987) Severly imparied adipsin expression in genetic and acquired obesity. Science 237:405-408 - Realisation that the fat cell is more than just a fat storage molecule
Steppan et al. (2001) The hormone resisten links obesity to diabetes. Nature 409:307-12 -First discovered resistin as a link between obesity and diabetes.
Fukuhara et al. (2005) Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science 307:426-30 - Discovery of another protein secreted from fat that may provide the link between obesity and diabetes
Zhang et al. (1994) Positional cloning of the mouse obese gene and its human homologue. Nature 372:425-32 - these authors first identified leptin
Schwartz et al. (2000) Central nervous system control of food intake. Nature 404:661-71 - detailing the effects of leptin on molecular pathways in the brain.
Youn BS et al. (2004) Plasma resistin concentrations measured by enzyme-linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab 89:150-6 PMID 14715842.
Al-Daghri NM et al. (2007) Adipocytokine profile of type 2 diabetics in metabolic syndrome as defined by various criteria. Diabetes Metab Res Rev; (Article online in advance of print) http://www3.interscience.wiley.com/cgi-bin/fulltext/114297091/HTMLSTART.
Norata GD et al. (2007) Plasma resistin levels correlate with determinants of the metabolic syndrome. Eur J Endocrinol 156:279-84.
Duman BS et al. (2007) Association of Resistin Gene 3'-Untranslated Region EX4-44G-->A Polymorphism with Obesity- and Insulin-Related Phenotypes in Turkish Type 2 Diabetes Patients. Rev Diabet.Stud. 4:49-55.
Lago F et al. (2007) The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev 18:313-25.
Anderson PD et al. (2007) Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab 92:2272-9.
- ↑ Note: Adipokines influence such processes as inflammation, control of appetite and energy balance, sensitivity to insulin, the metabolism of fats, and the growth of new blood vessels.