Electron orbital: Difference between revisions
imported>Paul Wormer |
imported>Paul Wormer (→Applications: Split off atomic orbital) |
||
| Line 59: | Line 59: | ||
==Applications== | ==Applications== | ||
Before the advent of electronic computers, orbitals (molecular as well as atomic) were used extensively in qualitative arguments explaining several properties of atoms and molecules. Orbitals still play this role in introductory texts and also in organic chemistry, where orbitals serve in the explanation of some reaction mechanisms, for instance in the [[Woodward-Hoffmann rules]]. | Before the advent of electronic computers, orbitals (molecular as well as atomic) were used extensively in qualitative arguments explaining several properties of atoms and molecules. Orbitals still play this role in introductory texts and also in organic chemistry, where orbitals serve in the explanation of some reaction mechanisms, for instance in the [[Woodward-Hoffmann rules]]. | ||
In modern computational quantum chemistry the role of atomic orbitals is different; they serve as a convenient expansion basis, comparable to powers of ''x'' in a Taylor expansion of a function ''f(x)'', or sines and cosines in a Fourier series. Below, the role of | In modern computational quantum chemistry the role of atomic orbitals is different; they serve as a convenient expansion basis, comparable to powers of ''x'' in a Taylor expansion of a function ''f(x)'', or sines and cosines in a Fourier series. Below, the role of molecular orbitals in qualitative theories will be discussed. | ||
===Atomic orbitals=== | ===Atomic orbitals=== | ||
Originally AOs were defined as approximate solutions of atomic Schrödinger equations, | |||
see the section ''solution of the atomic Schrödinger equation'' in [[atomic orbital]]'' for more on this. | |||
Recently, AOs are simply seen as convenient basis functions for quantum chemical computations. | |||
See the section ''AO basis sets'' in the article [[atomic orbital]] for more on the use of atomic orbitals in quantum chemistry. See [[Slater orbital]] | |||
for the explicit analytic form of orbitals and see [[Gauss type orbitals]] for a discussion | |||
of the type of AOs that are most frequently used in computations. | |||
===Molecular orbitals=== | ===Molecular orbitals=== | ||
| Line 139: | Line 124: | ||
If we apply the model to He<sub>2</sub>, which has four electrons, we find that we must place two electrons in the bonding orbital and two electrons in the ''antibonding MO'' σ<sub>''u''</sub>, with the total energy being 2β − 2β = 0. So, this simple application of molecular orbital theory predicts that H<sub>2</sub> is bound and that He<sub>2</sub> is not, which is in agreement with the observed facts. | If we apply the model to He<sub>2</sub>, which has four electrons, we find that we must place two electrons in the bonding orbital and two electrons in the ''antibonding MO'' σ<sub>''u''</sub>, with the total energy being 2β − 2β = 0. So, this simple application of molecular orbital theory predicts that H<sub>2</sub> is bound and that He<sub>2</sub> is not, which is in agreement with the observed facts. | ||
==References and notes== | ==References and notes== | ||
<references /> | <references /> | ||
Revision as of 04:22, 15 December 2007
In quantum chemistry, an electron orbital (or more often just orbital) is a synonym for a one-electron function. Here "orbital" is used as a noun. In quantum mechanics, the adjective orbital is often used as a synonym of "spatial" (as in orbital angular momentum), in contrast to spin (as in spin angular momentum).
The majority of quantum chemical methods expect that an orbital has a finite norm, i.e., that the orbital is normalizable (quadratically integrable), and hence this requirement is often added to the definition.
Outside of quantum chemistry an orbital is often seen as a wave function. This point of view is a narrowing of the quantum chemical definition, but not contradictory to it, provided a wave function is seen in this context as a solution of an (effective) one-electron Schrödinger equation. Indeed, in the past quantum chemists, too, distinguished one-electron functions from orbitals, the former being fairly arbitrary and the latter being solutions of certain one-electron Schrödinger equations. This distinction faded away from quantum chemistry, leading to the present definition as "normalizable one-electron function."
Usually on distinguishes two kinds of orbitals: atomic orbitals and molecular orbitals. Atomic orbitals are expressed with respect to one Cartesian system of axes centered on a single atom. Molecular orbitals (MOs) are "spread out" over a molecule. Usually this is a consequence of an MO being a linear combination (weighted sum) of atomic orbitals centered on different atoms.
Definitions of orbitals
Several kinds of orbitals can be distinguished.
Atomic orbital
See the article atomic orbital
Molecular orbital
The second kind of orbital is the molecular orbital (MO). Such a one-electron function depends on several vectors: rA1, rB1, rC1, ... where A, B, C, ... are different points in space (usually nuclear positions). The oldest example of an MO (without use of the name MO yet) is in the work of Burrau (1927) on the single-electron ion H2+. Burrau applied spheroidal coordinates (a bipolar coordinate system) to describe the wave functions of the electron of H2+.
Lennard-Jones[1] introduced the following linear combination of atomic orbitals (LCAO) way of writing an MO φ:
where A runs over Nnuc different points in space (usually A runs over all the nuclei of a molecule, hence the name molecular orbital), and i runs over the nA different AOs centered at A. The complex coefficients c Ai can be calculated by any of the existing effective-one-electron quantum chemical methods. Examples of such methods are the Hückel method and the Hartree-Fock method.
Spinorbitals
The AOs and MOs defined so far depend only on the spatial coordinate vector rA1 of electron 1. In addition, an electron has a spin coordinate μ, which can have two values: spin-up or spin-down. A complete set of functions of μ consists of two functions only, traditionally these are denoted by α(μ) and β(μ). These functions are eigenfunctions of the z-component sz of the spin angular momentum operator with eigenvalues ±½.
Spin atomic orbital
The most general spin atomic orbital of electron 1 is of the form
which in general is not an eigenfunction of sz. More common is the use of either
which are eigenfunctions of sz. Since it is rare that different AOs are used for spin-up and spin-down electrons, we dropped the superscripts + and −.
Spin molecular orbital
A spin molecular orbital is usually either
Here the superscripts + and − might be necessary, because some quantum chemical methods distinguish the spatial wave functions of electrons with different spins. These are the so-called different orbitals for different spins (DODS) (or spin-unrestricted) methods. However, many quantum chemical methods apply the spin-restriction:
Chemists express this spin-restriction by stating that two electrons [electron 1 with spin up (α), and electron 2 with spin down (β)] are placed in the same spatial orbital φ. This means that the total N-electron wave function contains a factor of the type φ(r1)α(μ1)φ(r2)β(μ2).
History of term
Orbit is an old noun introduced by Johannes Kepler in 1609 to describe the paths of the earth and the planets. The adjective "orbital" had (and still has) the meaning "relating to an orbit". When Ernest Rutherford in 1911 postulated his planetary model of the atom (the nucleus as the Sun, and the electrons as the planets) it was natural to call the paths of the electrons "orbits". Bohr, although he was the first to recognize (1913) orbits as stationary states of the hydrogen atom, used the word as well. However, after Schrödinger (1926) had solved his wave equation for the hydrogen atom (see this article for details), it became clear that the electronic "orbits" did not resemble planetary orbits at all. The wave functions of the hydrogen electron are time-independent and smeared out. They are more like unmoving clouds than like planetary orbits. As a matter of fact, the angular parts of the hydrogen wave functions are spherical harmonics and hence they have the same appearance as spherical harmonics. (See spherical harmonics for a few graphical illustrations).
In the 1920s electron spin was discovered, whereupon the adjective "orbital" started to be used in the meaning of "non-spin", that is, as a synonym of "spatial". In scientific papers of around 1930 one finds discussions about "orbital degeneracy", meaning that the spatial (non-spin) parts of several one-electron wave functions have the same energy. Also the terms orbital- and spin-angular momentum date form these days.
In 1932 Robert S. Mulliken[2] coined the noun "orbital". He wrote: From here on, one-electron orbital wave functions will be referred to for brevity as orbitals.[3] Then he went on to distinguish atomic and molecular orbitals.
Later the somewhat unfortunate term "spinorbital" was introduced for the product functions φ(r1)α(μ1) and φ(r1)β(μ1) in which φ(r1) has the tautological name "spatial orbital" and α(μ1) and β(μ1) are called "one-electron spin functions". The term "spinorbital" is unfortunate because it merges in one word the concepts of spin and orbital, which were distinguished carefully by early writers on quantum mechanics. For instance, one of the pioneers of theoretical chemistry, Walter Heitler, juxtaposes two-electron spin functions and two-electron orbital functions.[4] In the phrase "two-electron orbital function", Heitler uses orbital as an adjective synonymous with spatial (non-spin). Note, parenthetically, that Heitler does not refer to a "two-electron orbital", (there is no such thing as a two-electron orbital!). However, an inexperienced reader may easily and erroneously interpret the term "two-electron orbital function" as "two-electron orbital".
Applications
Before the advent of electronic computers, orbitals (molecular as well as atomic) were used extensively in qualitative arguments explaining several properties of atoms and molecules. Orbitals still play this role in introductory texts and also in organic chemistry, where orbitals serve in the explanation of some reaction mechanisms, for instance in the Woodward-Hoffmann rules. In modern computational quantum chemistry the role of atomic orbitals is different; they serve as a convenient expansion basis, comparable to powers of x in a Taylor expansion of a function f(x), or sines and cosines in a Fourier series. Below, the role of molecular orbitals in qualitative theories will be discussed.
Atomic orbitals
Originally AOs were defined as approximate solutions of atomic Schrödinger equations, see the section solution of the atomic Schrödinger equation in atomic orbital for more on this.
Recently, AOs are simply seen as convenient basis functions for quantum chemical computations. See the section AO basis sets in the article atomic orbital for more on the use of atomic orbitals in quantum chemistry. See Slater orbital for the explicit analytic form of orbitals and see Gauss type orbitals for a discussion of the type of AOs that are most frequently used in computations.
Molecular orbitals
In the hands of Friedrich Hund, Robert S. Mulliken, John Lennard-Jones, and others, molecular orbital theory was established firmly in the 1930s as a (mostly qualitative) theory explaining much of chemical bonding, especially the bonding in diatomic molecules.
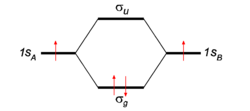
To give the flavor of the theory we look at the simplest molecule: H2. The two hydrogen atoms, labeled A and B, each have one electron (a red arrow in the figure) in an 1s atomic orbital (AO). These AOs are labeled in the figure 1sA and 1sB. When the atoms start to interact the AOs combine linearly to two molecular orbitals:
Earlier in this article a general expression for an LCAO-MO was given. In the present example of the σg MO of H2, we have Nnuc = 2, nA = nB = 1, χA1 = 1sA, χB1 = 1sB, cA1 = cB1 = Ng. With regard to the form of the MOs the following: Because both hydrogen atoms are identical, the molecule has reflection symmetry with respect to a mirror plane halfway the H—H bond and perpendicular to it. It is one of the basic assumptions in quantum mechanics that wave functions show the symmetry of the system. [Technically: solutions of the Schrödinger equation belong to a subspace of Hilbert (function) space that is irreducible under the symmetry group of the system]. Clearly σg is symmetric (is even, stays the same) under
while σu changes sign (is antisymmetric, is odd). The Greek letter σ indicates invariance under rotation around the bond axis. The subscripts g and u stand for the German words gerade (even) and ungerade (odd). So, because of the high symmetry of the molecule we can immediately write down two molecular orbitals, which evidently are linear combinations of atomic orbitals.
The normalization factors Ng and Nu and the orbital energies are still to be computed. The normalization constants follow from requiring the MOs to be normalized to unity. In the computation we will use the bra-ket notation for the integral over xA1, yA1, and zA1 (or over xB1, yB1, and zB1 when that is easier).
We used here that the AOs are normalized to unity and that the overlap integral is real
Applying the same procedure for Nu, we find for the two normalization factors
At this point one often assumes that S ≅ 0, so that both normalization factors are equal to ½√2.
In order to calculate the energy of an orbital we introduce an effective-one-electron energy operator (Hamiltonian) h,
Since A and B are identical atoms equipped with identical AOs, we were allowed to use
Likewise
The energy term β is negative (causes the attraction between the atoms). One may tempted to assume that q = −½ hartree (the energy of the 1s orbital in the free atom). This is not the case, however, because h contains the attraction with both nuclei, so that q is distance dependent.
If we now look at the figure in which energies are increasing in vertical direction, we see that the two AOs are at the same energy level q, and that the energy of σg is a distance |β| below this level, while the energy of σu is a distance |β| above this level. In the present simple-minded effective-one-electron model we can simply add the orbital energies and find that the bonding energy in H2 is 2β (two electrons in the bonding MO σg, ignoring the distance dependence of q). This model predicts that the bonding energy in the one-electron ion H2+ is half that of H2, which is correct within a 20% margin.
If we apply the model to He2, which has four electrons, we find that we must place two electrons in the bonding orbital and two electrons in the antibonding MO σu, with the total energy being 2β − 2β = 0. So, this simple application of molecular orbital theory predicts that H2 is bound and that He2 is not, which is in agreement with the observed facts.
References and notes
- ↑ J. E.Lennard-Jones, The Electronic Structure of some Diatomic Molecules;; Trans. Faraday Soc. vol 25, p. 668 (1929).
- ↑ R. S. Mulliken, Electronic Structures of Molecules and Valence. II General Considerations, Physical Review, vol. 41, pp. 49-71 (1932)
- ↑ Note that here, evidently, Mulliken uses the adjective "orbital" in the meaning of "spatial" (non-spin) and defines an orbital as, what is now called a "spatial orbital".
- ↑ W. Heitler, Elementary Wave Mechanics, 2nd edition (1956) Clarendon Press, Oxford, UK.









![{\displaystyle N_{g}=\left[2+2S\right]^{-1/2}\quad {\hbox{and}}\quad N_{u}=\left[2-2S\right]^{-1/2}.}](https://wikimedia.org/api/rest_v1/media/math/render/svg/6a8195f74219ed9fb2d24bded62392e7730dd0a1)


