Tenofovir: Difference between revisions
imported>David E. Volk m (→External links) |
imported>David E. Volk (chem infobox) |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
[[Image:Tenofovir structure.jpg| | |||
{{Chem infobox | |||
|align=right | |||
|image=[[Image:Tenofovir structure.jpg|center|thumb|200px]] | |||
|width=200px | |||
|molname=tenofovir | |||
|synonyms= TDF, PMPA | |||
|molformula= C<sub>9</sub>H<sub>14</sub>N<sub>5</sub>O<sub>4</sub>P | |||
|molmass= 287.2123 | |||
|uses=HIV/AIDS & Hep. B | |||
|properties=RT inhibitor, adenosine analog | |||
|hazards=see drug interactions | |||
|iupac= see chemistry section | |||
|casnumber=147127-20-6 | |||
}} | |||
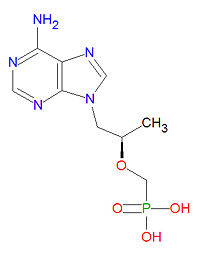
'''Tenofovir''' is a nucleotide analog [[reverse transcritase inhibitor]] (nRTI) [[antiviral drug]] used to treat [[HIV]]/[[AIDS]] and is in clinical trials for treatment of [[hepatitis B]] infection. The triphosphate from of the drug competes with the natural [[DNA]] [[nucleotide]] [[deoxyadenosine triphosphate]] (dATP) during DNA formation and it acts as a DNA chain terminator once incorporated because it lacks the normal [[deoxyribose]] sugar needed for connecting to the next DNA base. | '''Tenofovir''' is a nucleotide analog [[reverse transcritase inhibitor]] (nRTI) [[antiviral drug]] used to treat [[HIV]]/[[AIDS]] and is in clinical trials for treatment of [[hepatitis B]] infection. The triphosphate from of the drug competes with the natural [[DNA]] [[nucleotide]] [[deoxyadenosine triphosphate]] (dATP) during DNA formation and it acts as a DNA chain terminator once incorporated because it lacks the normal [[deoxyribose]] sugar needed for connecting to the next DNA base. | ||
== Chemistry == | |||
The IUPAC chemical name for tenofovir is [(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethylphosphonic acid and it has chemical formula C<sub>9</sub>H<sub>14</sub>N<sub>5</sub>O<sub>4</sub>P, giving it a molecular mass of 287.2123 g/mol. It is chemically similar to the natural neucleotide adenosine but lacks a ribose sugar unit. | |||
== Synonyms and brand names == | == Synonyms and brand names == | ||
Revision as of 12:58, 24 March 2008
|
| |||||||
| tenofovir | |||||||
| |||||||
| Uses: | HIV/AIDS & Hep. B | ||||||
| Properties: | RT inhibitor, adenosine analog | ||||||
| Hazards: | see drug interactions | ||||||
| |||||||
Tenofovir is a nucleotide analog reverse transcritase inhibitor (nRTI) antiviral drug used to treat HIV/AIDS and is in clinical trials for treatment of hepatitis B infection. The triphosphate from of the drug competes with the natural DNA nucleotide deoxyadenosine triphosphate (dATP) during DNA formation and it acts as a DNA chain terminator once incorporated because it lacks the normal deoxyribose sugar needed for connecting to the next DNA base.
Chemistry
The IUPAC chemical name for tenofovir is [(2R)-1-(6-aminopurin-9-yl)propan-2-yl]oxymethylphosphonic acid and it has chemical formula C9H14N5O4P, giving it a molecular mass of 287.2123 g/mol. It is chemically similar to the natural neucleotide adenosine but lacks a ribose sugar unit.
Synonyms and brand names
Synonyms
- Tenofovir disoproxil
- Tenofovir disoproxil fumarate
- D,L-Tenofovir
- TDF
- PMPA
Brand Names
- Apropovir
- Viread
External links
- Tenofovir - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank