Ciprofloxacin: Difference between revisions
imported>David E. Volk mNo edit summary |
imported>Milton Beychok (Undo revision 100471690 by David E. Volk (Talk) Undid inadvertant deletion by David Volk) |
||
| Line 3: | Line 3: | ||
'''Ciprofloxacin''' is a broad-spectrum antimicrobial carboxyfluoroquinoline used to treat [[urinary tract infections]], uncomplicated [[cystitis]], [[prostatitis]], lower [[respiratory tract infections]], [[sinusitis]], skin infections, bone and joint infections, infectious diarrhea, [[tyfoid fever]], cervical and urethral [[gonorrhea]], and post-exposure [[anthrax]]. Because it works by a different mechanism than other classes of [[antibiotic]]s, it can be used to treat some drug-resistant pathogens. | '''Ciprofloxacin''' is a broad-spectrum antimicrobial carboxyfluoroquinoline used to treat [[urinary tract infections]], uncomplicated [[cystitis]], [[prostatitis]], lower [[respiratory tract infections]], [[sinusitis]], skin infections, bone and joint infections, infectious diarrhea, [[tyfoid fever]], cervical and urethral [[gonorrhea]], and post-exposure [[anthrax]]. Because it works by a different mechanism than other classes of [[antibiotic]]s, it can be used to treat some drug-resistant pathogens. | ||
== Mechanism of actiion == | |||
The mechanism of action of [[quinolones]], including ciprofloxacin, is different from that of other antimicrobial agents such as [[beta-lactam]]s, [[macrolide]]s, [[tetracycline]]s, or [[aminoglycoside]]s; therefore, organisms resistant to these drugs may be susceptible to ciprofloxacin. There is no known cross-resistance between ciprofloxacin and other classes of antimicrobials. The bactericidal action of ciprofloxacin results from inhibition of the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV, which are required for bacterial DNA replication, transcription, repair, and recombination. | |||
== Chemistry == | |||
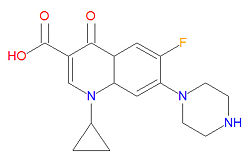
Ciprofloxacin's IUPAC chemical name is 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic acid and it has chemical formula C<sub>17</sub>H<sub>18</sub>FN<sub>3</sub>O<sub>3</sub> (MW = 331.3415 g/mol). | |||
== External links == | |||
* {{DailyMed}} | |||
* {{MedMaster}} | |||
* {{DrugBank}} | |||
Revision as of 12:37, 6 April 2009
Ciprofloxacin is a broad-spectrum antimicrobial carboxyfluoroquinoline used to treat urinary tract infections, uncomplicated cystitis, prostatitis, lower respiratory tract infections, sinusitis, skin infections, bone and joint infections, infectious diarrhea, tyfoid fever, cervical and urethral gonorrhea, and post-exposure anthrax. Because it works by a different mechanism than other classes of antibiotics, it can be used to treat some drug-resistant pathogens.
Mechanism of actiion
The mechanism of action of quinolones, including ciprofloxacin, is different from that of other antimicrobial agents such as beta-lactams, macrolides, tetracyclines, or aminoglycosides; therefore, organisms resistant to these drugs may be susceptible to ciprofloxacin. There is no known cross-resistance between ciprofloxacin and other classes of antimicrobials. The bactericidal action of ciprofloxacin results from inhibition of the enzymes topoisomerase II (DNA gyrase) and topoisomerase IV, which are required for bacterial DNA replication, transcription, repair, and recombination.
Chemistry
Ciprofloxacin's IUPAC chemical name is 1-cyclopropyl-6-fluoro-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic acid and it has chemical formula C17H18FN3O3 (MW = 331.3415 g/mol).
External links
- Ciprofloxacin - FDA approved drug information (drug label) from DailyMed (U.S. National Library of Medicine).
- Template:MedMaster
- Template:DrugBank