Units of radioactivity: Difference between revisions
imported>Howard C. Berkowitz |
imported>David Finn mNo edit summary |
||
| Line 1: | Line 1: | ||
{{subpages}} | {{subpages}} | ||
{{TOC|right}} | {{TOC|right}} | ||
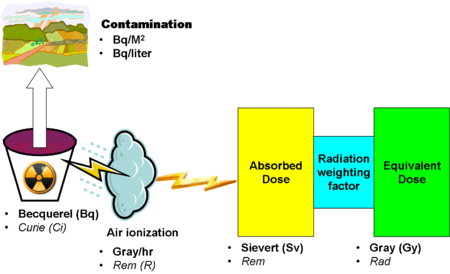
[[Image:RadiationUnitRelations.png|right|450px|thumb|Relations among units]] | |||
For the quantitative measurement of different aspects of [[radioactivity]], there are a substantial number of '''units of radioactivity'''. The number is relatively high because the aspects include the energy of a radiation source, as well as the effect of [[ionizing radiation]] in air, absorbed in arbitrary materials, and specific effects on biological systems. Doubling the number of units is the reality that they are defined both for the [[International System of Units]] (SI) and in [[U.S. customary units|"traditional" or "customary"]] units. | For the quantitative measurement of different aspects of [[radioactivity]], there are a substantial number of '''units of radioactivity'''. The number is relatively high because the aspects include the energy of a radiation source, as well as the effect of [[ionizing radiation]] in air, absorbed in arbitrary materials, and specific effects on biological systems. Doubling the number of units is the reality that they are defined both for the [[International System of Units]] (SI) and in [[U.S. customary units|"traditional" or "customary"]] units. | ||
Revision as of 06:47, 18 November 2011
For the quantitative measurement of different aspects of radioactivity, there are a substantial number of units of radioactivity. The number is relatively high because the aspects include the energy of a radiation source, as well as the effect of ionizing radiation in air, absorbed in arbitrary materials, and specific effects on biological systems. Doubling the number of units is the reality that they are defined both for the International System of Units (SI) and in "traditional" or "customary" units.
Properly interpreting the units can be quite complex. A given quantum of ionizing radiation will have different effects not only due to the type of radiation, which is considered in the computation of the units of biological effect, sieverts and rems, but also due to the energy level within a radiation type. Fast, medium-speed and slow neutrons, for example, have different biological effects.
Activity of a source of radiation
On arriving at an accident site, the Incident Commander tells the radiation survey specialist that a package of medical isotopes is on a crashed truck. It is labeled to contain 0.2 Ci or 7.4 x 109 Bq of Cs-137. She wants to know the risks it presents, and get advice on how to handle it.'[1] This is a practical example how single units do not fully characterize a hazard. The basic quantitative measurements define the amount of potential radioactivity in the container: two-tenths of a curie, or 200 millicuries, of cesium (Cs). This is equivalent to 7,400,000,000 Bq, or 7.4 GBq. To assess hazard, you must consider the isotope involved. Cesium-137, widely used in medicine and industry, and also a likely contaminant from a nuclear reactor accident, is a beta and gamma emitter with a half-life of 30 years. This half-life means it will decay to 0.1 curie in 30 years. Risk assessment requires a knowledge of the ionizing radiation emitted, and also the physical form of the isotope. Assume the package is intact, although some of the outer shielding may have torn off; there is no dispersal hazard. Beta and gamma radiation have different biological effects. They also have very different penetrating power. Beta particles are principally an internal hazard and a slight hazard to the outer skin. As long as the package contents are not dispersed, the beta aspect is relatively safe. Beta particles cannot travel, in air, for long distances. Even though the biological effect of gamma rays is less, the radiation can travel long distances, penetrate light shielding, and, as an external source, is a hazard to living things. "Practical steps that can be taken to reduce your internal risk to Cs-137 would include wearing anti-contamination clothing complete with face mask or respirator (if the responder is trained and respirator fitted.) Your exposure to the gamma emitter in Cs-137 can be reduced by relying on the exposure control methods of:
"Time spent in the radiation field may be lessened by rotating the crew. Unless you have a designated function, stay out of the radiation field. Put as much shielding between you and the radiation source as possible. The denser the material the better the shielding. For example, a fire truck may provide better shielding than a concrete block wall." |
As opposed to most of the other units, the SI unit becquerel[2] (Si) and the common unit curie (Ci) deal with the activity of the source, not the effects on radioactivity reaching its destination. Linked with area or volume measurements, these units are useful in giving a quantitative measurement of contamination of areas of the ground or volumes of water. When the source contains multiple isotopes, as with a reactor accident, it is most useful to state activity of each isotope.
Basic units
1 Bq = 1 event of radiation emission per second. Since this is a very small unit, common measurements are:
- 1 kBq = 103 Bq
- 1 MBq = 106 Bq
- 1 GBq = 109 Bq
The older unit, the curie (Ci), is equal to 37 GBq. Curies are large units, so common representations are
- 1 mCi = 10-3Ci
- 1 uCi = 10-6Ci
- 1 nCi = 10-9Ci
- 1 pCi = 10-12Ci
Linking the two sets of units,
Measuring contamination
Contamination of surfaces, such as the ground near a radiation release, is stated in Bq or Ci units per square metre. Contamination of water is expressed in Bg or Ci per liter.
Refining activity
Neither the Bq nor the Ci measure the energy of release from the source, only its rate. The energy is specified in electronvolts.
Half-life and decay reactions
Ionization of air
Roentgens measure the ionization of air from a radioactive source, but knowing that is less important than knowing other measurements. They measure exposure to radioactivity, but, since absorption and biological effects are more operationally important, R, and its SI approximation in Gy/Hr, are much less widely used in safety work.
Absorption in materials
Absorption units
Shielding effects
Biologic effects
Radiation damages cells by two classes of mechanism: direct and indirect. Direct damage results from chemical bonds directly absorbing ionizing radiation and being disrupted. Indirect damage comes from irradiation producing free radicals from water and other substances, and having these products chemically poison cells.[3]
The greatest direct damage comes from the most efficient linear energy transfer to complex molecules such as DNA.
| Radiation type | Energy level | Weighting factor (WR)) |
|---|---|---|
| Gamma rays and x rays | All | 1 |
| Beta particles | All | 1 |
| Neutrons | < 10 KeV | 5 |
| > 10 keV to 100 keV | 10 | |
| > 100 keV to 2 MeV | 20 | |
| > 2 MeV to 20 MeV | 10 | |
| > 20 MeV | 5 | |
| Alpha particles | All | 20 |
Linear energy transfer
Linear Energy Transfer (LET) provides one of the reasons that some types of radiation have more or less biological effect than others. Measured in keV/µm-kilo (electron volt per micrometer), it is the energy transferred per unit length of the track of radiation through tissue. [5]
- where:
- energy transferred by a charged particle of specified energy
- in traversing a distance of
References
- ↑ Radiological Emergency Response Independent Study, Emergency Management Institute, Federal Emergency Management Agency, January 1998, IS 301, pp. 2-1 to 2-2
- ↑ While the abbreviations of units are capitalized, the spelled-out names are not, to avoid confusion with the person they honor.
- ↑ FEMA, pp. 3-3 to 3-5
- ↑ Radiation - Quantities and Units of Ionizing Radiation, Canadian Centre for Occupational Health and Safety
- ↑ Mansoor Ahmed, Lecture 1: Interaction of Radiation with Biological Systems, Mammalian Radiation Biology Course, Sylvester Comprehensive Cancer Center, University of Miami Health System, p. 33
| Property measured | SI unit | Other unit | Notes |
|---|---|---|---|
| Rate of emission from a source |
|
|
|
| Air ionization by radiation |
|
|
|
| Absorbed dose |
|
|
|
| Biological equivalent dose |
|
|