Lysine: Difference between revisions
Jump to navigation
Jump to search

imported>Caesar Schinas m (Bot: Update image code) |
mNo edit summary |
||
| Line 2: | Line 2: | ||
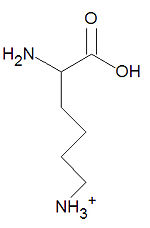
{{Image|Lysine stick figure.jpg|right|150px|'''Lysine''', a common amino acid.}} | {{Image|Lysine stick figure.jpg|right|150px|'''Lysine''', a common amino acid.}} | ||
'''Lysine''', abbreviated '''Lys''' or '''L''', is one of the twenty common [[amino acid]]s used by living organisms to build [[protein]]s. It is one of three positively-charge amino acids along with [[arginine]] and [[histidine]]. Because of the positive charge, lysine is mostly found exposed on the surface of a [[protein structure]]. DNA-binding proteins tend to have many lysines (and arginines) to enhance binding with the negatively-charged phosphate backbone of DNA molecules. | '''Lysine''', abbreviated '''Lys''' or '''L''', is one of the twenty common [[amino acid]]s used by living organisms to build [[protein]]s. It is one of three positively-charge amino acids along with [[arginine]] and [[histidine]]. Because of the positive charge, lysine is mostly found exposed on the surface of a [[protein structure]]. DNA-binding proteins tend to have many lysines (and arginines) to enhance binding with the negatively-charged phosphate backbone of DNA molecules.[[Category:Suggestion Bot Tag]] | ||
Latest revision as of 06:00, 14 September 2024
Lysine, abbreviated Lys or L, is one of the twenty common amino acids used by living organisms to build proteins. It is one of three positively-charge amino acids along with arginine and histidine. Because of the positive charge, lysine is mostly found exposed on the surface of a protein structure. DNA-binding proteins tend to have many lysines (and arginines) to enhance binding with the negatively-charged phosphate backbone of DNA molecules.