Gamma-aminobutyric acid
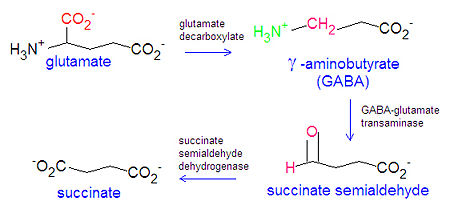
Gamma aminobutyric acid (GABA) or -aminobutyrate, is the major inhibitory neurotransmitter in the central nervous system.[1] GABA is produced from the amino acid glutamate through the action of the enzyme glutamate decarboxylase, and is inactivated by degradation to succinate in a two step mechanism involving the enzymes GABA-glutamate transaminase and succinate semialdehyde dehydrogenase.
Role in clinical pharmacology
GABA modulators have various roles.
Gamma-aminobutyric acid (GABA) agonists
Drugs that increase the effect or secretion of GABA are called GABAergic, such as baclofen.
Many sedatives work by increasing receptiveness of GABAA receptors.
Barbituates
Barbituates are GABAergic by increasing receptiveness of the GABAA receptors. Barbituates do this by increasing the duration of openings of channels in the cell membrane.[1]
- Phenobarbital
Gabapentin & Pregabalin
Gabapentin and pregabalinare both analogs that are agonists of GABA.
Nonselective BZ1 and BZ2 agonists
Benzodiazepines are also nonselective GABAergic by increasing receptiveness of the GABAA receptors. However, benzodiazepines do this by increasing the frequency of openings of channels in the cell membrane.[1]
Benzodiazepine receptors are BZ1 and BZ2.
BZ1 selective agonists
Cyclopyrrolones / Piperazines
Imidazopyridines
- Zolpidem (Ambien)
Pyrazolopyrimidines
- Zaleplon (Sonata; Starnoc). May have similar abuse potential to benzodiazepams.[2]
References
- ↑ 1.0 1.1 1.2 Katzung, Bertram G. (2006). Basic and clinical pharmacology. New York: McGraw-Hill Medical Publishing Division. ISBN 0-07-145153-6.
- ↑ Rush CR, Frey JM, Griffiths RR (1999). "Zaleplon and triazolam in humans: acute behavioral effects and abuse potential.". Psychopharmacology (Berl) 145 (1): 39-51. PMID 10445371.