Hepatocellular cancer
Hepatocellular cancer— commonly referred to as ‘hepatocellular carcinoma’, and sometimes ‘liver cancer’, ‘hepatic cancer’, or ‘hepatoma’—is a malignant growth of epithelial cells (i.e., a carcinoma) developing as a primary cancer within the tissues of the liver, as opposed to cancers having migrating (metastasizing) there from another organ or tissue.
A 2011 review in the New England Journal of Medicine summarizes the factors that increase the risk of someone developing hepatocellular carcinoma:
|
Major risk factors for hepatocellular carcinoma include infection [of the liver] with HBV [[[Hepatitis B virus]]] or HCV [[[Hepatitis C virus]]], alcoholic liver disease, and most probably nonalcoholic fatty liver disease. Less common causes include hereditary hemochromatosis, alpha1-antitrypsin deficiency, autoimmune hepatitis, some porphyrias, and Wilson’s disease. The distribution of these risk factors among patients with hepatocellular carcinoma is highly variable, depending on geographic region and race or ethnic group…Most of these risk factors lead to the formation and progression of cirrhosis, which is present in 80 to 90% of patients with hepatocellular carcinoma. [1] |
The highest incidence of hepatocellular carcinoma in patients with with cirrhosis of the liver occurs in association with Hepatitis C virus infection. High incidences also occur in patients with cirrhosis associated with Hepatitis B virus infection and hereditary hemochromatosis.[2] Other risk factors for hepatocellular carcinoma include obesity, type 2 diabetes mellitus, metabolic syndrome, and non-alcoholic liver disease—conditions all of which have a high coexistence relationship.[1] Moderate to high coffee consumption can reduce the risk of developing hepatocellular carcinoma in some high risk groups.[3] [4] Although the mechanism of coffee's risk reduction effect remains unelucidated, future studies of coffee's effect on the liver might help in elucidating the pathogenesis of hepatocellular carcinoma.
The World Health Organization's International Agency for Research on Cancer (IARC) reported on the the worldwide incidence and mortality of liver cancer in 2008:
|
Liver cancer is the fifth most common cancer in men (523 000 cases, 7.9% of the total) and the seventh in women (226 000 cases, 6.5% of the total), and most of the burden is in developing countries, where almost 85% of the cases occur, and particularly in men: the overall sex ratio male: female is 2.4. The regions of high incidence are Eastern and South-Eastern Asia, Middle and Western Africa, but also Melanesia and Micronesia/Polynesia (particularly in men)…There were an estimated 694 000 deaths from liver cancer in 2008 (477 000 in men, 217 000 in women), and because of its high fatality (overall ratio of mortality to incidence of 0.93), liver cancer is the third most common cause of death from cancer worldwide. The geographical distribution of the mortality rates is similar to that observed for incidence.[5] |
Diagnosis
The alpha-fetoprotein may be elevated; however, its positive predictive value is low and it may be elevated in patients with cirrhosis.[6]
Treatment
Hepatocellular cancer treatment information from the National Cancer Institute's Physician Data Query
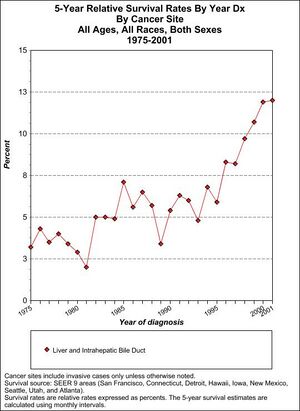
Prognosis
Staging information
Hepatocellular cancer staging information from the National Cancer Institute's Physician Data Query
Screening
"Surveillance is deemed cost-effective if the expected HCC risk exceeds 1.5% per year in patients with hepatitis C and 0.2% per year in patients with hepatitis B. Analysis of recent studies show that alpha-fetoprotein determination lacks adequate sensitivity and specificity for effective surveillance (and for diagnosis). Thus, surveillance has to be based on ultrasound examination. The recommended screening interval is 6 months" according to a clinical practice guideline by the American Association for the Study of Liver Diseases. [7] "In a mixed-aetiology cohort, the most effective surveillance strategy is to screen each patient with AFP assay and ultrasound imaging on a 6-monthly basis" according to a systematic review by the NIHR Health Technology Assessment programme (UK). [8] In a randomized controlled trial, the relative risk ratio of biannual alpha-fetoprotein and ultrasonography, as compared to no screening, for mortality from hepatocellular carcinoma was 0.6 and the relative risk reduction was 36.7%. In populations similar to those in this study which had a rate of risk as measured by the mortality from hepatocellular carcinoma of 0.1315% without treatment, the number needed to treat is 2070. [9]
References cited
- ↑ 1.0 1.1 El-Serag HB. (2011) Hepatocellular Carcinoma. Review Article. N.Engl.J.Med. 365(12):1118-1127. | 56 references.
- ↑ Fattovich G, Stroffolini T, Zagni I, (2004) Donato F. (2004) [http://dx.doi.org/10.1053/j.gast.2004.09.014 Hepatocellular carcinoma in cirrhosis: incidence and risk factors]. Gastroenterology 127:Suppl 1:S35-S50.
- ↑ Leung WW, Ho SC, Chan HL, Wong V, Yeo W, Mok TS. (2011) Moderate coffee consumption reduces the risk of hepatocellular carcinoma in hepatitis B chronic carriers: a case-control study. J Epidemiol Community Health 65:556-8.
- ↑ Johnson S, Koh WP, Wang R, Govindarajan S, Yu MC, Yuan JM. (2011) Coffee consumption and reduced risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Cancer Causes Control 22:503-10.
- ↑ GLOBOCAN Cancer Fact Sheet 2008.. Liver Cancer Incidence and Mortality Worldwide in 2008. International Agency for Research on Cancer.WHO.
- ↑ Tong MJ, Blatt LM, Kao VW (2001). "Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America.". J Gastroenterol Hepatol 16 (5): 553-9. PMID 11350553.
- ↑ Bruix J, Sherman M, American Association for the Study of Liver Diseases (2011). "Management of hepatocellular carcinoma: an update.". Hepatology 53 (3): 1020-2. DOI:10.1002/hep.24199. PMID 21374666. PMC PMC3084991. Research Blogging.
- ↑ Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M et al. (2007). "Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis.". Health Technol Assess 11 (34): 1-206. PMID 17767898. [e]
- ↑ Zhang BH, Yang BH, Tang ZY (2004). "Randomized controlled trial of screening for hepatocellular carcinoma.". J Cancer Res Clin Oncol 130 (7): 417-22. DOI:10.1007/s00432-004-0552-0. PMID 15042359. Research Blogging.
Cite error: <ref> tag with name "carr2010" defined in <references> is not used in prior text.
Cite error: <ref> tag with name "lau2008" defined in <references> is not used in prior text.
Cite error: <ref> tag with name "mcmasters2011" defined in <references> is not used in prior text.
Cite error: <ref> tag with name "kojiro2006" defined in <references> is not used in prior text.