Trypanosoma brucei
Articles that lack this notice, including many Eduzendium ones, welcome your collaboration! |
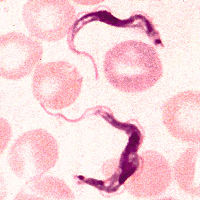
| Trypanosoma brucei | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Trypanosoma brucei |
Description and significance
Trypanosoma brucei is a unicellular parasitic organism with no true tissues. It belongs to the protista kingdom and therefore has cell structures that are similar to the cells of many eukaryotes. T. brucei has no definite shape, it is mobile and has a single flagellumfor locomotion.
T. brucei is often found in the rain forests,savannas, and rural areas of sub-saharan Africa. It is the cause of the disease trypanosomiasis, also called the African sleeping sickness. When this parasite infects cattles, it causes a disease called nagana. Failure in controlling the spread of the disease and enhancing local healthcare system have caused an increased impact of the parasite in both humans and animals living in underdeveloped regions of Africa. According to the World Health Organization, there are approximately 50,000-70,000 cases of trypanosomiasis found in Western and Central Africa. No one is immuned from this disease and it can be possible for one to get re-infected. Infection risk increases with the number of times a person gets bitten by the infected tsetse flies. Understanding the species of T. brucei will allow us to improve public health and cure for the sickness this organism causes in underdeveloped regions.
Genome structure
The Trypanosoma brucei genome was sequenced by the Institute for Genomic Researchand the Sanger Institute. This organism is made up of a two unit genome: a nuclear genome and a mitochondrial genome. The nuclear genome houses linear DNA molecules and are composed of 85% of the total cellular DNA. The nuclear genome has chromosomes that are divided into three types based on their sizes. There are 11 large chromosomes of 1-6Mb, 6 intermediate chromosomes of 300-600Kb, and 100 minichromosomes of 50-100kb. The intermediate chromosomes are found to play a role in antigenic variation, which is useful for T.brucei to escape the immune system of the host. The mitochondrial genome consists of the remaining 15% of the total cellular DNA. Inside the mitochondrial genome, the organism's kinetoplast is responsible for carrying 25-35 large circular DNA molecules called maxicircles and thousands of small circular DNA molecules called minicircules. Both the maxicircles and minicircles are organized into a network of a disk-shape structure, nucleoprotein disk, which is located at the base of the flagellum.
Cell structure and metabolism
Trypanosoma brucei has organelles including endoplasmic reticulum, golgi apparatus, lysosome, nucleus, and a large mitochondrion. Its mitochondrion houses a unique structure called kinetoplast, where DNA is located. T. brucei has a single flagellum for locomotion. The flagellum runs along the undulating membrane. As the flagellum oscillates, it produces a wavelike motion and pushes the cell along. The base of the flagellum is associated with the kinetoplast in a single large mitochondrion. The basal body plays a role in organizing spindles during mitosis. T.brucei is asexual and reproduces by binary fission through mitotic division. The basal body is the first to replicate, followed by the replication of the kinetoplast within the mitochondrion. As the daughter flagellum grows, the nucleus undergoes mitosis. After the mitochondrion divides, cytoplasm undergoes cytokinesis to form two identical cells.
Because of its animal-like cells, T. brucei has heterotrophic cells that require organic molecules for its source of energy. Studies have shown that it possesses some forms of energy producing enzymes. The organism acquires proline as a major energy source when it lives inside the gut of the tsetse fly. Since there is not much nutrients to be obtained from the gut of an insect, the parasite’s mitochondrion needs to carry out some type of metabolic pathways in order to generate sufficient energy. On the contrary, when nutrients become more abundant in mammalian host, the parasite can rely on the glucose from its host as its source of energy without having to undergo metabolic pathways to produce energy for its own use. This causes a severe malnutrition and rapid weight loss in the host.
Ecology
Life Cycle of Trypanosoma brucei
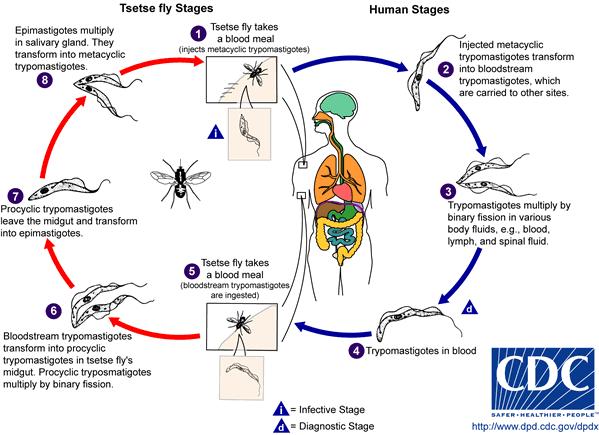
During the development of T. brucei, morphological changes play an important role in the organism’s life cycle betweenprocyclic stage and bloodstream stage. The parasite takes the form of procyclic trypomastigote while in the gut of the tsetse fly. Binary fission of the parasite takes place in the gut. Once the parasite leaves the gut, it transforms into epimastigote. As epimastigote reaches the fly’s salivary gland, it goes through a morphological change to form metacyclic trypomastigote, which is a form capable of infecting mammalian hosts. Aftermetacyclic trypomastigote is injected into a vetebrate host through the tsetse fly’s blood meal, the parasite circulates throughout the body of the host and changes into the form of bloodstream trypomastigotes, a form capable of infecting tsetse flies again. When a tsetse fly takes a blood meal on the infected host, it becomes infected and goes on to disperse the parasite into the next mammalian host. Migration of infected individuals from rural areas of Africa also spreads the parasite to other regions.
Pathology
The parasitic organism infects mammals by using tsetse flies as intermediate hosts. During a blood meal, the infected Tsetse fly injects the parasite into the mammalian host. The organism is then transmitted into the bloodstream of mammalian host and can be carried to the lymph and spinal fluid through circulation. T. brucei begins to replicate in the bloodstream. As the organism migrates to other sites of the body through blood fluids, it begins to invade other body tissues and the central nervous system of the host. This results in symptoms include enlarged lymph nodes, swollen tissues, fever, headache, insomnia, and mental deterioration in patients.
Treatments
There is currently no vaccine available to treat Trypanosomiasis. However, diagnostic tests are reliable methods to detect the presence of parasites. Analyzing blood smears, fluids from swollen lymph nodes and spines are examples of diagnostic methods available. Anti-trypanosomal agents are not highly recommendated often because of the significant potential side effects. Patients taking any of these medications will require close monitoring.
Current Research
1. "The developmental cell biology of Trypanosoma brucei"
The organization of cell structures and organelle positioning in T. brucei are specialized to govern morphological changes needed for the organism to adapt to environmental conditions in different hosts during various stages of the life cycle. The ability to survive in various environments requires a pre-programmed differentiation of the cell. This research article shows that the trypanosome life cycle interconnects with its whole cell. It puts together basic aspects of T.brucei’s high degree cell structures and their importance in regulating the organism’s cell cycle and developmental functions. Some of the important ideas can be summarized. The trypanosome cell is made up of microtubule cytoskeleton, which underlies the cell membrane and defines the cell shape of the organism. The flagellar pocket, flagellum, kinetoplast, mitochondrion and nucleus are located within the cytoskeleton corset. The small size of T. brucei allows rapid rate of protein trafficking from the inside of the cell to the cell surface. The kinetoplast repositions during T.brucei’s life cycle. Studies have shown that microtubule cytoskeleton extends and allows the posterior end of the cell grows to grow by 3 µm. The kinetoplast relocates toward the nucleus during DNA replication when the parasite differentiates from the stumpy form to the procyclic form. The complexity of the genomes and cell division can also be integrated to show how the cell structures are organized to adapt to the various stages of the life cycle.
Comparative genomic approach is used to identify genes that are associated with the motile flagella in Trypanosoma brucei. It was known that flagella play a multi-functional role in cell locomotion, nutrient uptake, and cell division. However, little was known about the regulation of flagellar beat in organisms. Here, T. brucei is used as an experimental system to study flagellar biology in eukaryotic cells. The function of flagella has extended to involve cell morphogenesis and host-parasite interactions in T.brucei. The motile flagella protein TbCMF is being studied in this research in hope to identify components of motile flagella that are evolutionarily conserved. The motile flagella protein TbCMF is composed of 30 novel genes. Mutants with knockout of these genes showed defects in motility. Ultrastructural analysis was used to identify the TbCMF genes and the results showed that TbCMF genes function to maintain connections between outer doublet microtubules. The results from this experiment give insights to how flagellum is critical for understanding mechanisms in disease pathogenesis and parasite development.
3. "Mitochondrial DNA Ligases of Trypanosoma brucei"
UCLA's research group has done a study on mitochondrial DNA ligases, which plays a major role in rejoining the gaps of the newly replicated minicircles in kinetoplast. Both sealing Okazaki fragments and and sealing the minicircles at the terminal step of the DNA replication require the enzyme DNA ligase. The models from the experiment confirmed the presence of two DNA ligase activities associated with the kinetoplast. The kinetoplastid genome was found to have DNA ligase genes LIG kα and LIG kß that not only are transported to the mitochondrion of the organism, but are also localized throughout the mitochondrial genome. The unique form of kinetoplast DNA indicates evolutionary divergence from higher eukaryotes. The gene LIG kß works with other repair enzymes and is most likely involved in joining Okazaki fragments on minicircles. A knockout of LIG kα gene has shown to inhibit ligation and stop the network in releasing covalently closed minicircles. This results in an accumulation of gapped minicircles, a reduction in size of the DNA network, and eventually a loss of DNA within the kinetoplast. The results from the experiment have suggested that LIG kα gene is responsible for the final sealing of gaps in minicircles at the origin of replication before cleavage of the double-size kinetoplast DNA network.
References
↑Bacteriol, J.(1992 February) “Mutual adjustment of glucose uptake and metabolism in Trypanosoma brucei grown in a chemostat”. Research Unit for Tropical Diseases, International Institute for Cellular and Molecular Pathology, Brussels, Belgium, 174(4): 1273–1279. Retrieved March 3, 2008, from PubMed Central Database.
↑Berriman, Matt. The Trypanosoma brucei Genome Project. The Wellcome Trust Sanger Institute. <http://www.sanger.ac.uk/Projects/T_brucei/>.
Black, Samuel J. (2001). World Class Parasites: Volume 1, The African Trypanosomes, First Edition. Kluwer Academic Publishers.
↑Centers for Disease Control and Prevention. 1600 Clifton Rd., NE, Atlanta, GA 30333. (800) 311-3435, (404) 639-3311. <http://www.cdc.gov>.
↑Downey, Nick. (2005 April) "Mitochondrial DNA Ligases of Trypanosoma brucei". American Society for Microbiology, Molecular Biology Institute and Department of Microbiology, Immunology, and Molecular Genetics, University of California, Los Angeles, California. 4(4): 765–774. Retrieved March 3, 2008, from PubMed Central Database.
↑"Life Cycle of Trypanosoma brucei." Chart. Retrieved March 3, 2008, from Centers for Disease Control and Prevention.
<http://www.dpd.cdc.gov/dpdx/HTML/TrypanosomiasisAfrican.htm>.
↑Matthew, Keith R. “The developmental biology of Trypanosoma brucei.” Institute of Immunology and Infection Research, School of Biological Sciences, University of Edinburgh, West Mains Road, Edinburgh, EH9 3JT, UK. Retrieved March 3, 2008, from Journal of Cell Science 118(2005):238-290. <http://jcs.biologists.org/cgi/content/full/118/2/283#SEC6>
Prescott, Harley, Klein. (2005). Microbiology, Six Edition. New York: McGraw Hill Companies (pp.569-573)
↑World Health Organization. Avenue Appia 20�CH - 1211 Geneva 27, Switzerland. +41 22 791 2111. <http://www.who.int/tdr/diseases/tryp/default.htm>