Borrelia burgdorferi
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is May 21, 2009. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
| Borrelia burgdorferi | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||
| Scientific classification | ||||||||||||||
| ||||||||||||||
| Binomial name | ||||||||||||||
| Borrelia burgdorferi |
Description and Significance
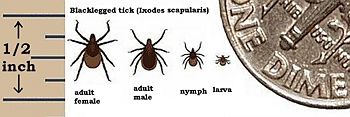
B. burgdorferi are gram negative spirochetes found primarily in North America and Europe. Its a double membraned, motile, spiral shaped microorganism that habitat the gut of deer ticks (Ixodes scapularis).[1] It's named after American scientist Willy Burgdorfer who in 1982 isolated and determined the bacteria to be the causative agent of Lyme disease.[2] Lyme disease is the most common tick borne disease in the US.[3][1]
Genome structure
B. burgdorferi has a small complex genome containing linear DNA, accompanied by up to 20 circular as well as linear plasmids. A study in 1997 found the linear DNA to be composed of 910,725 base pairs (average G+C content of 28.6%), and at least 17 linear and circular [[plasmid]s with a combined size of more than 533,000 base pairs. The small genome size of B. burgdorferi is associated with an apparent absence of genes for the synthesis of amino acids, fatty acids, enzyme cofactors, and nucleotides. The lack of biosynthetic pathways also explains why growth of B. burgdorferi in vitro requires serum-supplemented mammalian tissue-culture medium.
The chromosomes contains 853 genes encoding a basic set of proteins for DNA replication, transcription, translation, solute transport and energy metabolism, but no genes for cellular biosynthetic reactions. Of 430 genes on 11 plasmids, most have no known biological function.The biological significance of the multiple plasmid-encoded genes is not clear, although they may be involved in antigenic variation or immune evasion. [4][5]
| Number | Percent | |
|---|---|---|
| Total Number of all DNA molecules: | 18 | 100.00% |
| Total Size of all DNA molecules: | 1443725bp | 100.00% |
| Total number of linear DNA bases: | 910,725bp | 63.1% |
| Number of chromosomal G+C bases: | 256551bp | 28.5% |
| Number | Percent | |
|---|---|---|
| Total genes: | 853 | 100.00% |
| Protein coding genes: | 793 | 93% |
| Genes assigned a role category: | 502 | 59% |
| Genes not assigned a role category: | 51 | 6.26% |
| Hypothetical genes: | 102 | 12% |
Cell Structure
B. burgdorferi are long gram negative spirochetes (some researchers however claim that the organism does not exhibit properties of either gram positive or gram negative bacteria since they do not exhibit defining gram negative characteristics). Stained cells are visible under by dark-field or phase-contrast microscopy, but not visible by bright field microscopy. They are flexible helical cells and are much longer than they are wide. They usually have a length of 20-30um but a width of only 0.2-0.3um. The organism is motile with both rotational and translational movements, and the coiling of cells is regular. On the average, seven periplasmic flagella are located at each cell end, and these flagella overlap at the central region of the cell. There are multiple layers of membrane that surround the protoplasmic layer, cytoplasmic membrane, and the enclosed cytoplasmic contents. [1]
Metabolism
B. Burgdorferi are classified as being chemoorganotropic, and derive energy by metabolizing organic molecules. The organism is microaerophilic; they require oxygen to survive, but only at low concentrations. Too much or lack of oxygen can be harmful to them. They cannot synthesize their own amino acids, fatty acids, enzyme cofactors, and nucleotides. They also happen to be catalase negative; they are unable to break down hydrogen peroxide. It's possible to cultivate the organism in vitro, but it requires a very complex growth medium since it lacks the ability to synthesize various necessary compound (see genome structure). The medium used to grow Borrelia burgdorferi is called Barbour-Stoenner-Kelly (BSK) medium. It contains over thirteen ingredients in a rabbit serum base.
Ecology
The optimal growth temperature is 34° to 37°C, and the organism has a generation time of 11 to 12 hours at 35°C. Their life cycle is directly linked to their host, and they depend on arthropod vectors (ticks) to infect mammals. They reside in infected mammals as well as in the gut of ticks. They multiply when there is enough blood ingested by the tick to support bacterial growth.
The vector tick, Ixodes scapularis, undergoes a two year life cycle beginning when the female drops from her animal host to lay eggs in the soil during the spring. Very small larvae emerge during the summer and attach to a passing small animal (such as mouse) to feed on its blood. If the small animal is infected with the spirochete, the larva become infected as they feed. the fed larvae drop back to the ground and molt into nymphs. the nymphs wait until next year's warm weather to search for another host to feed on before becoming adult ticks. They prefer the white-footed mouse, but will take any warm-blooded host that happens along. At this point, the infected nymphs can infect new animal hosts (including human). The fed nymph falls back to earth to molt, and emerge as an adult tick. The adult has one more blood feeding before it mates by late spring, and infected nymphs carry the infection into adulthood. Male ticks die following mating, while female ticks die after egg laying, and thus, completing the life cycle (Daniels and Falco 1989; Kantor 1974).
Pathology
Borrelia Burgdorferi is the is the causative agent of Lyme disease, and is transmitted to mammalian hosts by tick vectors. Lyme Disease is a multisystem inflammatory disease that affects the skin in the early stage and then spreads to the joints, nervous system and may eventually affect the organ systems. The bite of an infected nymph or adult tick can only transmit Lyme disease after it attaches to the host for thirty six to forty eight hours. This is due to the initial lag time, and the generation time of B. Burgdorferi, which happens to be 12 hours (see ecology). Spirochetes waiting in the mid-gut of the tick only begin to multiply as the tick starts its blood meal. Then they migrate to the salivary glands where they are discharged into the host with the tick's saliva. It takes several hours before a large enough infectious dose of spirochetes can penetrate the new host.
Initial sign and sympotoms of Lyme disease include an appearance of a rash, which has the appearance of being solid red or bull’s eye rash with flu like symptoms.
Application to Biotechnology
Current Research
References
- ↑ 1.0 1.1 1.2 Johnson, Russell C.; George P. Schmid, Fred W. Hyde, A. G. Steigerwalt, Don J. Brenner (1984). "Borrelia burgdorferi sp. nov.: Etiologic Agent of Lyme Disease". Int J Syst Bacteriol 34 (4): 496-497. DOI:10.1099/00207713-34-4-496. Retrieved on 2009-04-29. Research Blogging.
Cite error: Invalid
<ref>tag; name "description" defined multiple times with different content - ↑ Burgdorfer, W; AG Barbour, SF Hayes, JL Benach, E Grunwaldt, JP Davis (1982). "Lyme disease-a tick-borne spirochetosis?". Science 216 (4552): 1317-1319. DOI:10.1126/science.7043737. Retrieved on 2009-04-29. Research Blogging.
- ↑ MD, Alan G. Barbour (1996-04-01). Lyme Disease: The Cause, the Cure, the Controversy, 1. The Johns Hopkins University Press. ISBN 0801852455.
- ↑ Fraser, Claire M.; Sherwood Casjens, Wai Mun Huang, Granger G. Sutton, Rebecca Clayton, Raju Lathigra, Owen White, Karen A. Ketchum, Robert Dodson, Erin K. Hickey, Michelle Gwinn, Brian Dougherty, Jean-Francois Tomb, Robert D. Fleischmann, Delwood Richardson, Jeremy Peterson, Anthony R. Kerlavage, John Quackenbush, Steven Salzberg, Mark Hanson, Rene van Vugt, Nanette Palmer, Mark D. Adams, Jeannine Gocayne, Janice Weidman, Teresa Utterback, Larry Watthey, Lisa McDonald, Patricia Artiach, Cheryl Bowman, Stacey Garland, Claire Fujii, Matthew D. Cotton, Kurt Horst, Kevin Roberts, Bonnie Hatch, Hamilton O. Smith, J. Craig Venter (1997-12-11). "Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi". Nature 390 (6660): 580-586. DOI:10.1038/37551. ISSN 0028-0836. Retrieved on 2009-04-27. Research Blogging.
- ↑ Casjens, S; N Palmer, R van Vugt, W M Huang, B Stevenson, P Rosa, R Lathigra, G Sutton, J Peterson, R J Dodson, D Haft, E Hickey, M Gwinn, O White, C M Fraser (2000-02). "A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi". Molecular Microbiology 35 (3): 490-516. ISSN 0950-382X. Retrieved on 2009-04-27.