Law of multiple proportions (chemistry)
Nineteenth century chemist, John Dalton's (1766-1844) law of multiple proportions: If two elements — elements Y and Z, say — can form multiple different compounds, the ratio of the weights of any one element in the different compounds — element Z, say — when it combines with a fixed weight of another — element Y, say — will compute as ratios of small integral (whole) numbers — viz., Z:Y = 2:1 or 3:1 or 3:2, etc.
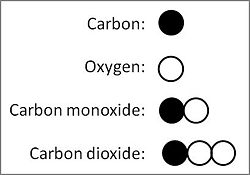
Using modern values for the atomic masses of elements, the existence of the two compounds of carbon (C) and oxygen (O), carbon monoxide (CO) and carbon dioxide (C02), offers a simple example to illustrate the law of multiple proportions. Carbon, the element of fixed mass in the two compounds, which today chemists take as having an atomic mass of 12, combines with two different masses of oxygen, namely 16 (atomic mass of oxygen) in carbon monoxide, 32 (sum of atomic mass of two oxygen atoms) in carbon dioxide. Those two masses of oxygen in the two compounds with a fixed mass of carbon relate to each other in the ratio 16-to-32, which equals 1-to-2, a ratio of two whole small numbers. Dalton would have determined the actual weights in grams of oxygen in the two compounds, adjusting them to a fixed weight of carbon, and calculated the ratio of oxygen in the two compounds therefrom. Thus, empiricaly observed, 10 grams of carbon combines with 13.3 grams of oxygen to form carbon monoxide, and 26.6 grams of oxygen to form carbon dioxide, the ratio 13.3:26.6 = 1:2 for oxygen in the two compounds.
For another example, consider the elements nitrogen, N, and oxygen O. The element oxygen occurs in the compounds NO (nitric oxide) and NO2 (nitric dioxide). The ratio of oxygen weights (1:2) in those compounds contains the small whole numbers 1 and 2.
Note that in modern chemistry the concept "number of atoms" replaces "weight", used by Dalton. Now chemists say that the ratio of numbers of O-atoms in different NOx compounds (or in different N2Ox compounds) is expressible as a ratio of small whole numbers.[1]
Bernard Jaffe, in his popular book on the history of chemistry, Crucibles, The Story of Chemistry: From Ancient Alchemy to Nuclear Fission, describes the history of the law of multiple proportions:
While working on the relative weights of the atoms, Dalton noticed a curious mathematical simplicity. Carbon united with oxygen in the ratio of 3 [parts by weight] to 4 [parts by weight] to form carbon monoxide, that poisonous gas which is used as a fuel in the gas-range. Carbon also united with oxygen to form gaseous carbon dioxide in the ratio of 3 [parts by weight] to 8 [parts by weight]. Why not 3 to 6, or 3 to 7? Why that number 8 which was a perfect multiple of 4 [a ratio of 2-to-1]? If that were the only example, Dalton would not have bothered his head. But he found a more striking instance among the oxides of nitrogen, which Cavendish and Davy had investigated. Here the same amount of nitrogen united with one, two and four parts of oxygen to form three distinct compounds. Why these numbers which again were multiples of each other? He had studied two other gases, ethylene and methane, and found that methane contained exactly twice as much hydrogen as ethylene. Why this mathematical simplicity? [2]
Thinking atomistically, Dalton literally figured out the answer, using the figures, or symbols, he had invented for an atom of each of the known elements: (see accompanying illustration.)
If, as Dalton's atomic theory proposed, elements consisted of atoms differing in weight among elements but of equal weights for the atoms of a given element, and compounds consisted of atoms of different elements, then when a fixed bulk weight of one element combined to form different compounds with another element, the ratio of the bulk weights of that other element in the different compounds must relate as ratios of small whole numbers, as those bulk weights reflect the accumulated weight of the atoms of that other element in single particles of the compounds, which themselves relate as ratios of small whole numbers.
Jaffe reports that the Swedish chemist, Jöns Jacob Berzelius (1779-1848), a contemporary of Dalton, stated Dalton´s explanation as:
In a series of compounds made up of the same elements, a simple ratio exists between the weights of one and the fixed weight of the other element.
And in a letter to Dalton, Berzelius wrote:
….this Law of Multiple Proportions was a mystery without the atomic hypothesis.[2]
By extension of Dalton's law of multiple proportions, the subscripts m, n, k, ... in a compound AmBnCk⋅⋅⋅ are integral numbers (integers). In other words, the law applies to the ratio of the differing elements in a given compound as well as the ratio of the same element in differing compounds.
The law of multiple proportions helped lead Dalton to his theory that an element consists of atoms of the same weight and that the atoms of different elements differ, inasmuch as when the weights of element O, say, when it forms different compounds with a fixed weight of element N, say, the differing weights of O in the differing compounds relate as the ratio small whole numbers. It also helped lay the foundation for writing formulas for chemical compounds.
Dalton assigned the mass of the lightest element, hydrogen, 1, as the unit of atomic mass, enabling him to determine the relative atomic weights of different elements — relative to the unit weight of hydrogen. The law of multiple proprtions upholds the validity of the theory of matter's nature as comprising atoms of different masses, and therefore of different shapes and sizes.
References and notes cited in text as superscripts
- ↑ Eight molecular manipulable models of different compounds of solely nitrogen and oxygen, illustrating the law of multiple proportions. USC Department of Chemistry.
- ↑ 2.0 2.1 Jaffe B. (1976) Crucibles, The Story of Chemistry: From Ancient Alchemy to Nuclear Fission. 4th Edition. Dover Publications, Inc: New York. ISBN 0-4S6-2J342•j. Full-Text of Chapter VII, Dalton: A Quaker Builds the Smallest of Worlds.