Glucostatic theory of appetite control
For the course duration, the article is closed to outside editing. Of course you can always leave comments on the discussion page. The anticipated date of course completion is 01 February 2011. One month after that date at the latest, this notice shall be removed. Besides, many other Citizendium articles welcome your collaboration! |
In the early twentieth century, a link was first made between blood glucose and appetite that led to the glucostatic theory of appetite control. In 1916, Carlson had suggested that plasma concentrations of glucose could serve as a signal for both meal initiation (low levels) and meal termination (high levels) [1]. But it was not until the 1950's that Mayer put forward the glucostatic hypothesis. Originally it was thought that a rise in plasma glucose, for example after a meal, was sensed by neurons in the hypothalamus. These neurons which included “glucoreceptors” then signalled for meal termination. Glucose was thus thought of as a satiety factor [2].
This theory has been debated for many years. While many studies appear to support Mayer’s hypothesis, many others do not, and compelling evidence has yet to be found. The theory, which was popular in the 1950s, was losing support by the 1980s. At this time, scientists were beginning to think that the control of appetite was a more complex mechanism that would have to depend on the integration of a number of signalling pathways. The glucostatic theory was not abandoned altogether, as it was still thought to be important for short-term appetite control, but newly discovered peptides such as leptin became more likely candidates for long-term control.
Physiological background
Glucose homeostasis must be finely regulated by the absorption of food and the flow of recently stored energy substances through different metabolic pathways. Especially for brain glucose, it has to be supplied continuously from the blood stream since there is no storage for sugar available in the brain. It is known that changes in glucose level elicit complex neuroendocrine responses that restore blood sugar levels to the optimum range [3]. It is traditionally believed that different regions of the forebrain; particularly the hypothalamus and the brain stem have important centres which are responsible for monitoring blood glucose level and regulating feeding [4]. However, Ritter R. C. et al. claimed that glucoreceptor cells are located in the hindbrain. This means that the glucose sensing cells have direct access to the central nervous system and could elicit immediate responses to retain the physiological norm [5]. They also explained that the catecholamine neurons in the hindbrain help mediating responses to glucose deficiency by linking glucoreceptor cells to forebrain and spinal neurons. This enables us to stimulate behavioural and hormonal responses that elevate blood sugar level. These include increased food intake, adrenal medullary secretion, corticosterone secretion and suppression of estrous cycles. Complex behaviours involved in activities such as detection and identification of food are mainly regulated by the forebrain. Her studies suggest that the hind brain mediates the motivation for these activities via the neuronal circuit activated by some of the glucose sensing cells. They hypothesized that the signals detected by the glucoceptors are projected to the hypothalamus via norepinephrine and epinephrine neurons in the hind brain. This motivation circuit would have engaged the physical sign of energy deficiency with these behaviours [6].
Short-term control of appetite and satiety
The Glycaemic Index (GI) is a measure of the effects of glycaemic carbohydrates on postprandial blood glucose levels. Foods that are digested rapidly to produce a sharp rise in blood glucose are classified as high-GI, whereas foods that are digested and absorbed slowly have a low-GI. It has been hypothesised that the GI of food plays an important role in regulating appetite.
Recent studies suggest that low GI diets prolong satiety and thereby reduce food intake. Pre-adolescent children (9 to 12 years-old) given low-GI breakfasts ate less lunch and showed a lower hunger rating than children who had high-GI breakfasts. The weight or sex of a child did not alter the effect of the GI of the breakfasts on lunch intake. [7] In obese adolescents, low-GI meals are associated with a lower insulin response than high-GI meals. The time intervals between meals were longer in low-GI test meal group, indicating that low-GI meals increased satiety. [8] Another study by Arumugam et al. investigated the effect of variations in postprandial glycaemia and insulinaemia on subjective appetitive sensations in overweight and obese women. They modulated the rate of ingestion of a glucose beverage to examine the postprandial effects of high and low-GI meals. Sharp peaks followed by rapid decrease in glucose and insulin levels to below baseline were observed in subjects with rapid consumption while relatively stable glucose and insulin levels were seen in subjects with slow consumption. Higher hunger ratings and prospective intake were reported by the subjects with rapid consumption than those with slow consumption after meals demonstrating the positive relationship between blood glucose concentrations and satiety [9].
On the other hand, Flint et al. argue that after ingestion of various GI meals by healthy young male participants showed no association between glycaemic response and postprandial fullness though it may reduce energy intake in a subsequent meal. In contrast, insulinaemic response after the meal seemed to be positively correlated with postprandial satiety [2].
Thus, these short-term studies suggest that glycaemic and insulinaemic responses are implicated in appetite and satiety. The relatively early decline of blood glucose level to below the baseline after high-GI meal seems to play a role in initiating hunger and appetite.
The Long-Term Control of Feeding and Energy Balance
The glucostatic hypothesis represents a physiological control system that fits the criteria for controlling short-term energy consumption. Few studies have looked at the glucostatic theory and the effect of varying GI foods in the long-term, possibly due to their reliance on participants and the lack of experimental control in such a long time frame. A study by Alfenas and Mattes looked at the long-term implications on appetite of consuming high- or low-GI foods over multiple days and weeks. Their findings suggested there were no significant differences in both glycaemic and insulinemic responses, and, in hunger, fullness, or desire to eat [10].
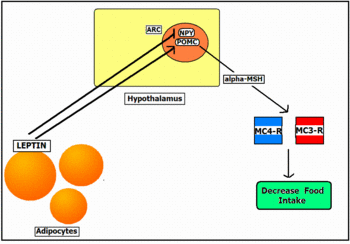
In contrast with the short-term, the long-term control of feeding appears to occur via a different mechanism involving the secretion of a hormone Leptin, from fat (adipose) tissue, and its action on the central nervous system. The effects of leptin were first discovered in the 1950’s, although leptin itself was not discovered until 1994. The concentration of leptin in the circulation is proportional to the amount of fat in the body, and it alters body weight by either inhibiting the intake of food or by triggering hyperphagia. Once secreted, Leptin crossed the blood-brain-barrier via a receptor-mediated transport system [11]. Its receptors are highly expressed in the hypothalamus in areas such as the Arcuate Nucleus (ARC), and in the brain stem nuclei; these are both areas that are known to control feeding and energy balance. The binding of Leptin to these receptors influences the peptide systems, Pro-opiodmelanocortin (POMC) and Neuropeptide Y (NPY). POMC neurons in the arcuate nucleus produce the peptide alpha-MSH, an appetite suppressant. NPY is a potent feeding stimulant. An increase in leptin levels inhibits peptides, such as NYP, and causes an increase in alpha-MSH, which binds to Melanocortin 3 receptors (MC3-R) and Melanocortin 4 receptors (MC4-R), causing a decrease in feeding and regulating the long-term control of energy balance [12]. This type of leptin signalling is illustrated in Figure 2.
The role of insulin and ghrelin in the glucostatic theory
It has been proposed that both ghrelin and insulin play important roles in appetite, hunger and the glucostatic hypothesis. Increase in blood glucose and insulin level seems to prolong satiety by stimulating the release of leptin and suppressing ghrelin. However, studies have produced conflicting results and how these peptides relate to the glucostatic theory still remains unclear.
Insulin
Insulin, a peptide hormone, is required by cells in order to take up and use glucose from the blood. Studies have shown that insulin is correlated with satiety and food intake. It was found that in the short-term insulin is involved in limiting postprandial appetite [2]. However, glycaemic responses appear to be more important for this. Another study found that in obese and overweight women serum insulin levels were negatively correlated with subjects hunger ratings. They were also positively correlated to fullness. Again it was found that glucose had a bigger part to play in this than insulin. Both these studies suggest that, although other factors appear to be important, insulin may play a role in appetite control.
Conversely, other studies have found no relationship between insulin and appetite. Because of this, it is uncertain how important insulin is in the control of appetite.
Ghrelin
It is now commonly accepted that ghrelin, a peptide produced in the stomach, stimulates appetite. It has been proposed that this peptide is involved in glucostatic signalling. Ghrelin levels peak before a meal or when a meal is expected. In rats it has been found that insulin-induced hypoglycaemia up regulates ghrelin mRNA expression. Ghrelin secretion is also inhibited by high glucose levels. This suggests that the cells which secrete ghrelin may be sensitive to changes in plasma glucose. This mechanism may play an important role in regulating energy metabolism.
When ghrelin is deleted, blood glucose is reduced and insulin levels are increased. This loss of ghrelin increases the ability of insulin to suppress glucose production. These findings show that ghrelin is important for glucose homeostasis. However, in a study by Sun et al. it was found that these effects were not related to changes in food intake or weight. As with insulin, there is little conclusive evidence suggesting an important role for ghrelin in the glucostatic hypothesis. There are, however, some studies have shown links between the two.
Conclusion
At present, though there has been several studies supporting the glucostatic theory in a short term, the GI of food modulating appetite and satiety in a longer period of time is uncertain. The relationship between insulin and anorexigenic leptin and orexigenic ghrelin is unclear and whether their levels are affected by GI values are difficult to simply conclude. One of the major factors that could influence these results is individual differences in the rate of digestion. Thus, this area of the field needs to be further long term investigation, encompassing the individual variance to be able to conclude that GI value is a valid predictor of appetite and satiety.
References
- ↑ Mobbs CV et al. (2005). "Impaired glucose signaling as a cause of obesity and the metabolic syndrome: the glucoadipostatic hypothesis.". Physiol Behav 85: 3-23. DOI:10.1016/j.physbeh.2005.04.005. PMID 15924903. Research Blogging.
- ↑ 2.0 2.1 2.2 Flint A, Møller BK, Raben A, Sloth B, Pedersen D, Tetens I et al. (2006). "Glycemic and insulinemic responses as determinants of appetite in humans.". Am J Clin Nutr 84 (6): 1365-73. PMID 17158418.
Cite error: Invalid
<ref>tag; name "pmid17158418" defined multiple times with different content Cite error: Invalid<ref>tag; name "pmid17158418" defined multiple times with different content - ↑ Ritter S, Dinh TT, Li AJ (2006). "Hindbrain catecholamine neurons control multiple glucoregulatory responses.". Physiol Behav 89 (4): 490-500. DOI:10.1016/j.physbeh.2006.05.036. PMID 16887153. Research Blogging.
- ↑ MAYER J (1955). "Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis.". Ann N Y Acad Sci 63 (1): 15-43. PMID 13249313.
- ↑ Ritter RC, Slusser PG, Stone S (1981). "Glucoreceptors controlling feeding and blood glucose: location in the hindbrain.". Science 213 (4506): 451-2. PMID 6264602.
- ↑ Singer LK, Ritter S (1996). "Intraventricular glucose blocks feeding induced by 2-deoxy-D-glucose but not mercaptoacetate.". Physiol Behav 59 (4-5): 921-3. PMID 8778887.
- ↑ Warren JM et al. (2003). "Low glycemic index breakfasts and reduced food intake in preadolescent children.". Pediatrics 112: e414. PMID 14595085.
- ↑ Ball SD et al. (2003). "Prolongation of satiety after low versus moderately high glycemic index meals in obese adolescents.". Pediatrics 111: 488-94. PMID 12612226.
- ↑ Arumugam V et al. (2008). "A high-glycemic meal pattern elicited increased subjective appetite sensations in overweight and obese women.". Appetite 50: 215-22. DOI:10.1016/j.appet.2007.07.003. PMID 17714828. Research Blogging.
- ↑ Alfenas RC, Mattes RD (2005). "Influence of glycemic index/load on glycemic response, appetite, and food intake in healthy humans.". Diabetes Care 28 (9): 2123-9. PMID 16123477. [e]
- ↑ Wynne K, Stanley S, McGowan B, Bloom S (2005). "Appetite control.". J Endocrinol 184 (2): 291-318. DOI:10.1677/joe.1.05866. PMID 15684339. Research Blogging.
- ↑ Ahima R, Osei SY (2004). "Leptin and appetite control in lipodystrophy.". J Clin Endocrinol Metab 89 (9): 4254-7. DOI:10.1210/jc.2004-1232. PMID 15356017. Research Blogging.