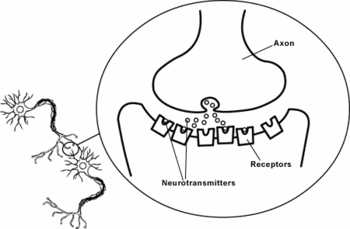
Synapse
A synapse is a specialized junction through which neurons transmit information to each other or to non-neuronal cells such as muscles or glands. At chemical synapses, neurons transmit information using chemical messengers, called neurotransmitters. At electrical synapses, the presynaptic and postsynaptic cell membranes are connected by channels that are capable of passing electrical current.
The word "synapse" comes from "synaptein" which Charles Scott Sherrington and his colleagues coined from the Greek syn- ("together"( and haptein ("to clasp").
Synapses are crucial to the biological computations that underlie perception and thought. They also provide the means through which the nervous system connects to and controls the other systems of the body.
History
In the second half of the 19th century, there were two vigorously debated hypotheses about the structure of the brain. Proponents of the cell theory considered that the brain consisted of independent units (neurons), while others thought of as a continuous web-like reticulum (a syncytium). The debate was resolved when the new technique of electron microscopy revealed the cellular structure of the brain, and of the contacts between them. Ramon y Cajal, using the Golgi staining method, described differences between classes of neurons and precise connections between them. Later, electron microscopy and other techniques have showed that some neurons possess channels, called connexons, which permit electrical transmission between cells.
The first investigations of the properties of synapses were conducted on nerve-muscle synapse by Dale in 1936.[1]
Structure
The nerve cell, transmitting the signal, is called the presynaptic cell; its target, receiving the signal, is called the postsynaptic cell. The presynaptic cell transmits the signal from the swollen tips of its axon’s branches, called presynaptic terminals, also called the synaptic boutons. The part of synapses where neurotransmitter is released is called the active zone. The presynaptic terminals usually end on the postsynaptic neuron’s dendrites, soma, or, less often, on the axon. The presynaptic cell does not make an anatomical contact with the postsynaptic cell since two cells are separated by a space, the synaptic cleft, which is about 20- 40 nm wide.[2] Immediately behind the postsynaptic membrane is an elaborate complex of interlinked proteins called the postsynaptic density. Proteins in the postsynaptic density serve a myriad of roles, from anchoring and trafficking neurotransmitter receptors into the plasma membrane, to anchoring various proteins.
Chemical synapses can vary in size and shape. Under the electron microscope, synapses are seen either as symmetric or asymmetric. Asymmetric synapses are responsible for excitatory inputs, while symmetric ones transmit inhibitory inputs.
The number of synapses that one neuron can make varies greatly. The average neuron makes about 1000 synapses and can receive as many as 10,000 synapses; the Purkinje cell of the cerebellum receives up to 100,000. Early in development, neurons make many synapses, later the number declines, reaching the level of adulthood. The process is called activity dependent synapse elimination, when active synapses are reinforced and inactive ones are eliminated. The number of synapses can change even in the adult animals, contributing to synaptic plasticity. The adult human brain can have from 1015 to 5 × 1015 synapses.
Signaling across chemical synapses
Within the presynaptic nerve terminal, vesicles containing neurotransmitter sit "docked" at the synaptic membrane and ready to release the neurotransmitter. The release of neurotransmitter, the exocytosis, is triggered by the arrival of a nerve impulse (or action potential). The arriving action potential produces an influx of calcium ions through voltage-dependent, calcium-selective ion channels. Calcium ions then trigger a biochemical cascade which results in vesicles fusing with the presynaptic membrane and releasing their contents into the synaptic cleft. Vesicle fusion is driven by the action of a set of proteins, known as SNAREs. The membrane added by this fusion is later retrieved by endocytosis and recycled for the formation of fresh neurotransmitter-filled vesicles.
After a neurotransmitter is released into the synaptic cleft, it can bind ionotropic or metabotropic receptors at the postsynaptic membrane. Ionotropic receptors are themselves ion channels; when a neurotransmitter binds to these receptors, the receptor conformation changes. As a result, ion channels open and the ions flow in or out of the cell, changing the membrane potential. The resulting change in voltage is called a postsynaptic potential.
Whether an effect is excitatory or inhibitory depends on what type(s) of ion channel conduct the postsynaptic current, which in turn is a function of the type of receptors and neurotransmitter employed at the synapse. The two most common neurotransmitters in the mammalian central nervous system are glutamate and GABA. Glutamate is an excitatory neurotransmitter, and can depolarise cells via its actions at any of three types of ionotropic receptor: kainate receptors; AMPA receptors; and NMDA receptors; activation of any of these allows the flow of K+, Na+ and sometimes Ca2+ into the postsynaptic cell. When GABA binds to GABA-A receptors, chloride channels open in the postsynaptic cell membrane. In adult neurons this usually results in chloride entering the postsynaptic cell and thus hyperpolarising it, but embryonic neurons and some adult cell types have a relatively high intracellular chloride concentration, and in these cases GABA actions may cause chloride to leave the cell, resulting in a depolarisation. Thus GABA can act as an excitatory neurotransmitter, but is mainly thought of as the most abundant inhibitory neurotransmitter in the adult.
Other important neurotransmitters in the mammalian CNS include purines, acetylcholine, serotonin, dopamine, serotonin and norepinephrine.
Synaptic transmission through ionotropic receptors is fast, taking just a few milliseconds to transmit the signal.
Neurotransmitter may also bind to metabotropic receptors, which are usually G-protein coupled receptors. When these receptors are activated, they produce intracellular second messengers, which subsequently may activate ion channels through phosphorylation. Such responses can last for several minutes.
Regulation of synaptic transmission
The last step in the synaptic transmission is the elimination of the neurotransmitter from the synaptic cleft. This removal prevents the desensitization of the post-synaptic receptors and ensures that succeeding action potentials will elicit the same size postsynaptic potential. The neurotransmitter can be removed because of diffusion, degradation or uptake by glial cells or nerve terminals.
The action of glutamate, glycine, GABA, dopamine, norepinephrine and 5-HT is terminated by uptake of transmitters by specific transport proteins, called transporters. Transporters neurotransmitter into nerve terminals can be packed into the vesicles and released again. ACh molecules in the synaptic cleft are degradated by a specific enzyme acetylcholinesterase, ATP is terminated by hydrolysis. Neuropetides are removed from the synaptic cleft by diffusion. Neurotransmitter degradation or removal is a prompt process, whereas diffusion is a slower one.
The necessity of removal of neurotransmitters and desensitization of receptors and ion channels means that the strength of a synapse may in effect diminish as a train of action potentials arriving in rapid succession. The phenomenon is called frequency dependence of synaptic transmission. The nervous system exploits this property for computational purposes, and can tune its synapses through such means as phosphorylation of the proteins involved. The size, number and replenishment rate of vesicles also are subject to regulation, as are many other elements of synaptic transmission.
Synaptic plasticity
The efficacy of chemical transmission is not fixed, but depends on the ongoing activity in the synapse. These changes can be a short-term, lasting from milliseconds to minutes, and long-term, lasting hours or days.[3] Short periods of synaptic activation can lead in facilitation, depression or augmentation of neurotransmitter release. All these processes change the amount of neurotransmitter released, last seconds and have a presynaptic origin. Posttetanic facilitation also has a presynaptic origin, but it usually lasts for tens of minutes. Repetitive stimulation can result in long-term potentiation (LTP) or long-term depression (LTD) of synaptic strength. The mechanisms of LTP and LTD have both presynpatic and postsynaptic origins.
Integration of synaptic inputs
Generally, if an excitatory synapse is strong, an action potential in the presynaptic neuron will trigger another action potential in the postsynaptic cell; whereas at a weak synapse the excitatory post-synaptic potential (EPSP) will not reach the threshold for action potential initiation. In the brain, however, each neuron typically forms synapses with many others, and likewise each receives synaptic inputs from many others. When many synapses of a neuron receive excitatory inputs at the same time, the neuron may generate an impulse even though the synapses are weak. This process is known as spatial summation. If only one synapse is active, but it receives many impulses in a short period of time, impulses are also summated. This process is called temporal summation. On the other hand, a pre-synaptic neuron releasing an inhibitory neurotransmitter such as GABA can cause inhibitory postsynaptic potential in the postsynaptic neuron, decreasing its excitability and therefore decreasing the neuron's likelihood to fire an action potential. In this way the output of a neuron may depend on the inputs of many others, each of them may have a different degree of influence, depending on the strength of its synapse and the location on the neuron. John Carew Eccles performed some of the important early experiments on synaptic integration, for which he received the Nobel Prize for Physiology or Medicine in 1963. Complex input/output relationships form the basis of transistor-based computations in computers, and are thought to figure similarly in neural circuits.
Comparison with electrical synapses
An electrical synapse forms a narrow gap between the pre- and postsynaptic cells, known as a gap junction. At gap junctions, cells approach within about 3.5 nm of each other[4], a much shorter distance than the 20 to 40 nm distance that separates cells at chemical synapses.[2] As opposed to chemical synapses, the postsynaptic potential in electrical synapses is not caused by the opening of ion channels by chemical transmitters, but by direct electrical coupling between both neurons. Electrical synapses are therefore faster, current spreads instantaneously, whereas chemical synapses have a delay of about 1 ms. Electrical synapses conduct equally to both directions, while at the chemical synapse the impulse is send only from pre- to postsynaptic neuron. Electrical synapses are found throughout the nervous system, yet are less common than chemical synapses. Electrical and chemical transmission can coexist at a single synapse.[3] Such combined synapses were first found avian ciliary ganglion cells.[5]
References
- ↑ Dale HH et al. (1936) J Physiol 86:353-80
- ↑ 2.0 2.1 Hormuzdi SG et al. (2004). "Electrical synapses: a dynamic signaling system that shapes the activity of neuronal networks". Biochim Biophys Acta 1662: 113–37. DOI:10.1016/j.bbamem.2003.10.023. PMID 15033583. Research Blogging.
- ↑ 3.0 3.1 Martin A al. et (2001). From neuron to brain. Sunderland, Mass: Sinauer Associates. ISBN 0-87893-439-1.
- ↑ Jessell TM et al. (2000). Principles of neural science. New York: McGraw-Hill. ISBN 0-8385-7701-6.
- ↑ Martin AR, Pilar G (1963) J Physiol 168:443-63