Triazole
|
| |||||||
| triazole | |||||||
| |||||||
| Uses: | antifungal | ||||||
| Properties: | basic | ||||||
| Hazards: | |||||||
| |||||||
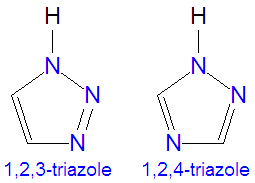
The triazoles are two isomers, namely 1,2,3-triazole or 1,2,4-triazole, with the formula C2H3N3. They are aromatic ring compounds that are similar to the azoles pyrazole and imadazole, but they would have an additional nitrogen atom in the ring structure. Like the azoles, triazoles are used in many antifungal drugs and fungicides, and the triazole-based drugs are more selective for fungi than mammalian cells compared to the azole-based antifungal compounds. Today, most substituted triazoles are produced by so-called click chemistry pioneered by K. Narry Sharpless and others (see below).
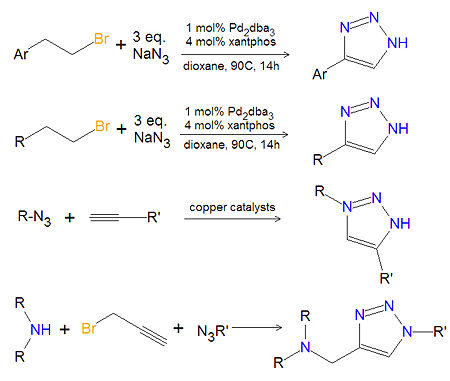
Chemistry of substituted 1,2,3-triazoles
Most substituted triazoles are synthesized by using one of several click chemistry methods. One method uses paladium catalysts to couple alkenyl halides and sodium azide to product 4-substituted-1,2,3-triazoles[1]. This reaction also works with aromatic alkenyl halides. Click chemistry between azides and terminal aklynes, catalyzed by various copper reagents, can be used to synthesize 3,5-disubstituted-1,2,3-triazoles[2],[3].
A variety of 1,4-disubstituted-1,2,3-triazoles can be made economically and in an environmentally friendly manner, by reacting a secondary amine, propargyl halides and an azide together in water, under atmospheric oxygen with a copper catalystic.[4]. Another method of 1,4-disubstituted-1,2,3-triazoles (not shown) uses no solvent. In this method, the cycloaddition of alkyl azides with enol ethers provides triazoles that are not amendable to synthesis using the coupling of azides with alkynes.[5] A variety of other synthetic methods can be found at organic-chemistry.org[6].
References
- ↑ J. Barluenga, C. Valdes, G. Beltran, M. Esribano, and F. Aznar (2006). "{{{title}}}". Angew. Chem. Int. Ed. 45: 6893-6896.
- ↑ (2002) "{{{title}}}". Angew. Chem. 114: 2708-2711.
- ↑ B.H. Lipshutz, B. R. Taft (2006). "{{{title}}}". Angew. Chem. Int. Ed. 45: 8235-8238.
- ↑ Yan, Zhao, Fan, Liu and Liang (2005). "{{{title}}}". Tetrahedron 61: 9331-9337.
- ↑ D.R. Rogue, J. L. Neill, J. W. Antoon, E. P. Stevens (2005). "{{{title}}}". Synthesis 2005: 2497-2502.
- ↑ 1,2,3-triazole synthesis. Retrieved on 2008-05-17.