Wheat streak mosaic virus
| Wheat Streak Mosaic Virus | ||||||||
|---|---|---|---|---|---|---|---|---|
 | ||||||||
| Virus classification | ||||||||
|
Description and Significance
Wheat Streak Mosaic Virus (WSMV) cannot live and reproduce by itself. WSMV is transferred through wheat curl mite to the crop. This mite is an arthropod. It is so extremely small in size that it cannot be seen with the naked eyes. Overall, the mite serves as a vector for the WSMV to get to the wheat. The wheat is the favorite food source and place to live for both the mite and the WSMV. As the mites reproduce, the WSMV is also passed down to the mites’ progeny. The peak reproduction of mites occurs when the temperature is warmer in spring around 75-80˚F. Here, the WSMV is also replicated and reproduced progressively as the mites population multiply in number.[1]
Massive numbers of mites are carried to the wheat field through the wind. WSMV from the mites then would infect the wheat. WSMV is the cause of the wheat disease.[2] The symptoms result from the WSMV infection are very distinct from other virus. The wheat's leaves turn yellowish and have narrow bands. There are blotches on the leaves with different patterns. The normal growth of the wheat is hindered. If the infection is more severe, the leaves can turn completely brown and die off.[3] The total yield of the wheat drops down significantly. If the infection is severe, the loss can be up to 100%.[4]
Distribution
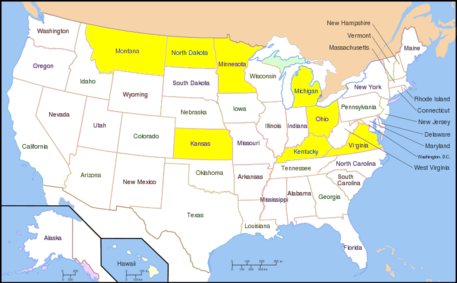
In the US, the WSMV infection is heavily found in the central plains: North Dakota, Kentucky, Michigan, Minnesota and Ohio.The fall and spring wheat field suffer the detrimental effects significantly. Now the virus infection is moderate in control as the scientists understand more about the causes, effects and mechanisms of virus transmission. The most prevalent infection occurred in 1987-1988. Recently, WSMV also found in Virginia in 2000.[5]
In the world, according to the data collected by US Department of Agriculture, the other countries that are also known to suffer from WSMV disease are Canada, Jordan, Romania, Australia, Yugoslavia, and Russia.
Genome structure
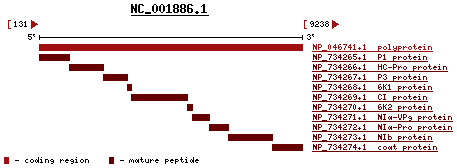
The WSMV is positive, linear, single-strand RNA (ssRNA). According to the International Committee on Taxonomy of virus, the complete genome of WSMV has 9384 nucleotides. The G+C content is 44%. The percent of coding is 97%. The sequence only codes for one long polypeptide. This long polypeptide has both structural and non-structural proteins.[6] In the image provided from pubmed reference page on the left, the long polynucleotide sequence code for 10 proteins: P1 protein, HC-Pro protein, P3 protein, 6K1 protein, CI protein, 6K2 protein, NIα-VP9 protein, NIα-Pro protein, NIb protein and coat protein.
According to three research articles from Virology, Plant Journal and Phytopathology, some of the functions of these genes have been revealed. P1 and HC-Pro have proteinase activity.[7] In addition, NIa is proteinase as well but it also codes for VPg sequences. CI is the inclusion body protein and shared by a type of RNA helicase. NIb is RNS-dependent RNA polymerase. The coat protein has carboxy terminal. Functions of the P3, 6K1 and 6K2 are still unknown. So far P1 and Hc-Pro gene have been the two genes of WSMV that have the greatest number of studies. Some researchers even knock out the HC-Pro gene and observe the effects on the virus.[8]
Furthermore, there are many different strains of the same WSMV. Some are milder than others. The strains are sorted out based on the damages and severity of symptoms on different wheat types. So far, three strains have been isolated from US, Eurasia and Mexico. Studies have shown that these strains are mosaic and differ in there nucleotide sequence by 10 to 20%. Five isolated strains of WSMV have their nucleotide sequences studied completely by researchers. These five strains are AF057533, AF285169, AF285170, AF454454 and AF454455. Their detailed sequences are deposited in the bank for further study and use in the future.[9]
Virus structure
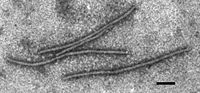
The virus is in group IV, family Potyviridae, genus Tritimovirus. The virus is simple in structure. It has helical capsid. It is naked and doesn't have envelope. The capsid has spiral arrangement in a long tube, about 700 nm in length, and 15nm wide. The single strand RNA is enclosed within the helical capsid. Researchers usually use uranyl acetate and phosphotungstate to stain the virus for electron microscopy.[10]
The WSMV had similar appearances with other three viruses are agrpyron mosaic, ryegrass mosaic and hordeum mosaic virus. It was thought that WSMV had a close interconnection with those viruses. However, recent studies have revealed that there is no relationship found between those viruses.
Characteristics of wheat cells that are infected with WSMV
The wheat cell bears many inclusions in the cytoplasm. The nucleus is distorted in shape. The inclusions do not have any rigid shape. They are varied in appearance. The inclusions are involved with viral proteins. There is an increased reproduction rapidly in number of membranes and ribosome’s. As the infections go on and progress, the cells is twisted out of shape and the organelles are disintegrated.
Other possible hosts for WSMV
The virus not only attacks all sorts of wheat but also the other cereal plants. The other cereal plants are oats, barley, rye, maize, millets and sorghum. Wild grass is also affected. If cereal plants and wild grass are grown in very close distance to the infected wheat crop, they can catch the mites by wind and develop WSMV quickly. However, young wheat is still the number one favorite host of mite and WSMV. Mites and WSMV only attack the other cereal plants when the wheat crops are severe damaged, grown old, degraded in nutritional values.
How to identify the wheat infected with WSMV
It’s hard to differentiate the normal, healthy wheat from the early infected wheat that hasn’t had any apparent, visible symptoms yet. Plant pathologists usually use electron microscope or perform Enzyme Linked Immunosorbent Assay test (ELISA). These tests were conducted in the labs by entomologists and plant pathologists. In ELISA test, the sap of infected plants is collected in antivirus serum. The result of the test is then compared with the known sap with WSMV. The more similarities between the reference and unknown sap, the more likely the plant is infected with WSMV.
Some of other viruses also have early infection symptoms similar to WSMV infection. Another test that plant pathologists also can use to confirm the presence of WSMV is the Immunosorbent Electron Microscopy (ISEM). This method involves in incubation of antiserum coated grids with virus suspensions. Different viruses have different trapping results.
Ecology
The virus's favorite natural habitat is wheat. It uses the wheat curl mite as a vector. The relationship between the virus and the wheat is parasitism. The virus takes advantage of the wheat and grows at the cost of the wheat's development.[11]
The image on the left basically summarizes the infection cycle of the virus in spring and winter wheat. Winter wheat crop starts to have early infection in the fall. The virus travels along with mites. The mites originate from infected spring wheat crop, volunteer wheat and grass. These mites are carried by the wind to the seeding winter wheat. Young, fresh, seedling wheat are best-loved food of both the mites and the viruses. As time goes on and the disease progresses, the winter wheat crop is stunted and yellowed and dies prematurely.
The infection in spring wheat depends on the mites that survive from the winter wheat. Infection starts even earlier if the spring crop is grown near the infected winter wheat crop. The volunteer wheat plays an important role between winter and spring wheat crop. Volunteer wheat usually grows in between the young and infected fields. It bears the reservoir of mites and acts as a mean for the mites to jump to the young wheat field nearby. Infected volunteer wheat from winter gives rise to infection in wheat in spring, and infected volunteer wheat in spring crop in turn is responsible for infection of wheat in the next fall.
Furthermore, the grass and corn crops nearby are also a reservoir of infection. The mites also can live in these hosts when all the wheat crops have deprived all nutritive values and died out. The mites stay in here and leave for the wheat later if the nearby wheat field are seeding and growing. Beside damage to the wheat, the virus is also indirectly gives a detrimental effect to the human. The wheat infection outbreak due to WSMV has given a great loss of money to the wheat food manufacture industry. Fall infection can result in up to 50% loss. Loss of spring infection is around 20%. An example is the 1995 outbreak in Montana. The loss was $35 million. Even now, in Kansas, WSMV is responsible for the loss of $20 million annually.
Pathology
The WSMV only infects cereal plants and hinders the growth of wheat. It does not infect angiosperms and humans.
Recommendation for control measures
There is no type of wheat that exists that shows resistance to the WSMV. Therefore, control measures have been suggested to avoid serious crop loss. In addition, it is important to understand that control virus infection also relates to control of mite population. Since the virus cannot travel by itself, it needs a vector to get to the host. Without the vector as its motor vehicle, the virus will not be present in the new crop and spread out infection.[12] Some of the control methods are:
1)Delaying seeding & plant winter and spring wheat at different days: Delaying seeding has been shown to be helpful. Mites reproduce maximally when the temperature is warm. As the mites reproduce, the virus also passes along the progeny. Without the host, mites only can survive up to two days. Delaying seeding when the weather is a little bit colder somewhat will control mite transmission because the cold weather is unfavorable to the mites' being. This is most helpful for winter wheat crop when the temperature drops down in winter time.
Delaying seeding also helps with spring wheat. In the mid-west states as Minnesota, North Dakota, the wet and rainy days in the summer makes the mites to stay where they are. Mites only can be carried by the wind when the it is a dry day. Wet weather helps prevent mite's mobilization. In turn, it also helps us to prevent virus spreading out. In some states, the planting days are set for each local county. These official planting days set up in local office make each of the next step easier to implement in a time-line manner.
2)Destroying volunteer wheat and grass hosts: In North West Minnesota, the researchers have advised the farmers to control the volunteer wheat first before they plant new wheat crop. This method targets in breaking the mite and virus transmission to the new, young, growing crop. If there are no volunteer wheat around, the mites have less chance to jump around.
3)Don't plant susceptible young wheat crops in proximity to the infectious crops to prevent mite's movement: Infection occurs with higher frequency and greater extent when the young crops grow in very close distance to the infectious crops. The young, nutritious wheat crops are always the target of the mites. Since one cannot control the direction of wind, this method somewhat decreases risk factors of mite mobilization. If space is not limited, one should plant the two crops as far as possible.
4)Using chemical agents: Chemical reagents are used to kill volunteer wheat and grass, since these are the temporary hosts where the mites can stay on. It is important to know that chemical control only helps to prevent mite's reproduction and mobilization. Once the wheat crops have been infected, the chemical method is not an effective method to implement anymore. The common chemical reagent use is Glycophosate [N(phosphonomethyl) glycine]. This herbicide is expensive. It works well to kill the plants but it doesn't work right away. The volunteer wheats and grass do not die overnight but several weeks later. The effectiveness of this herbicide also depends on the weather. It works best when the weather is rainy. During these rainy days, mite population have been shown to reduce significantly. When the days are dry, this herbicide doesn't work much. Even now, there are cases that the volunteer wheat has died down but mite's population maintains and continues to spread out. This is probably due to the mites' adaptation, so their resistance increases over time.[13]
There are always pros and cons for using the chemical control. One side is that the chemical should be effective to kill the mites, but at the same time the hazardous level also should be monitored in proper moderation. If the herbicide is too hazardous, it may harm the crops and indirectly harm us, the wheat's consumers. The chemical use and dosage also should be a particular concern when using this method.
Overall, every method has its own strengths and weaknesses. Controlling virus infection or mite mobilization should use the combination of all the methods. The plans should be conducted in a time-line manner and includes all the elimination of mite's reservoirs.
Application to Biotechnology
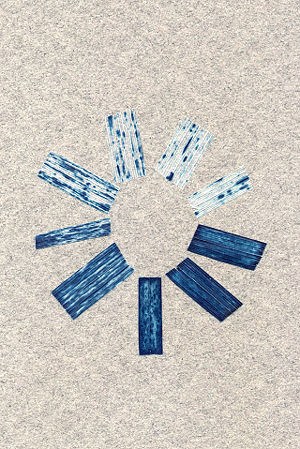
The image above shows several pieces of the wheat leaves. Some are blue. Some are lighter in color. Blue stain is used as a functional marker of the new genes. Blue pieces suggest the presence of the foreign genes in the wheat. These new genes are successfully delivered by WSMV as a gene vector.
WSMV has been known as the cause for the loss of wheat industry. However, now the research team in Wheat, Sorghum and Forage Research Unit, University of Nebraska, has used the virus as a vector to transfer the new genes into the wheat genome. The two leading researchers of the team, Drake Stenger and Roy French, have successfully decoded all 9,384 nucleotides of its RNA genome. In their laboratory, the researcher use WSMV as a vector to deliver foreign genes into the wheat plants. They equip the virus with two new genes of bacterial enzymes and let the virus attack the wheat. The foreign genes are tracked by using blue stain. The presence of the chemical blue stains in wheat indicates that the new virus's genes are already in the wheat.[14]
In the picture in the left, the blue pieces are taken out from the wheat leaves. Blue leaves suggest the presence and expression of the foreign genes. These new genes are delivered by the WSMV vector. According to French and Stenger, this new way allow them to get faster results. Usually they use the plant's callus for the gene's insertion. This conventional method takes from 6 to 9 months to see the results. By using the virus as a vector, it only takes about 4 to 5 days. This amazing method is due to the virus's exceptional ability to insert to the wheat tissue and expresses its gene expression.
The research team aims to use the virus to study the wheat's gene sequences that are still unknown until now. They also aim to create new wheat with improved traits and resistance to the virus infection. Through the virus as a gene vector, the team also suggests the possibility to block the virus's genes that are detrimental to the wheat. If these genes are not able to express, the virus would become harmless.
If these aims are able to carry out successfully, the powerful gene vector of virus promises the new turn over for the bioengineering and agriculture's research.[15]
Current Research
"Diallel Analysis of Wheat streak mosaic virus Resistance in Winter Wheat" by Frederic Hakizimanaa, Amir M. H. Ibrahim, Marie A. C. Langhama, Scott D. Haleyb and Jackie C. Rudd.
Even though the recommended control measures were carried out by farmers, the WSMV infection was still prevalent. The purpose of this study was to perform diallel cross to yield the resistant cultivars for WSMV. Diallel cross had been used previously. It was performed to determine the genetic contribution of each parent to the offspring. In this study, the researchers used nine winter wheat genotypes. These winter wheat were known for their various levels of resistance. In February 2000, the experiment was conducted randomly with four replications. One replications served as a control. Each genotype was planted in a 3:1:1:1 ratio. Each pot had the same environmental conditions. The pots then were place in the greenhouse. The experiment was repeated again in April 2000 with similar design. The researchers then performed inoculation on seedling wheat with WSMV. The research did the disease assessment by rating the infected plants with a scale from 0-5. Nine winter wheat types were used in the study. Their names were NE91648, Jagger, 2137, Dawn, SD93267, Harding, KS93WGRC27, Roughrider, and Sage. Results showed that the planting environment in greenhouse had more WSMV infection than the field experiment. The Roughrider and Sage had a high level of susceptibility. NE 91648 also had significant susceptibility. From this study, the best winter wheat types that could be used to defense against WSMV were Harding, Dawn and SD93267. Genotypes and the environmental conditions also played an important role in the cross.[16]
“Random mutagenesis of wheat streak mosaic virus HC-Pro: non-infectious interfering mutations in a gene dispensable for systemic infection of plants” by Drake C. Stenger, Brock A. Young and Roy French.
Previous researches had suggested that helper component-proteinase (HC-Pro) played a major role in the systemic infection by WSMV and other related virus in the family Potyviridae. Point mutations that did not affect proteinase activity in Potyviruses, limited its replication and infectivity. However, cleavage of WSMV HC-Pro had shown that deletion did not have any changes in systemic infectivity and host range. The goal of the study was to evaluate point mutations in WSMV and its infectivity, its ability to establish primary infection foci and its proteinase activity in vitro. The methods were conducted in a series of four steps. First, the researchers constructed HC-Pro mutants. They used an infectious clone of WSMV-Sidney 81. Then PCR was used to amplify the HC-Pro region. The copies of clones were collected. These clones had the substitution mutations on their HC-Pro genes. Second, they conducted the infectivity assays to confirm the infection status with RT-PCR. Third was the GUS assays; the GUS reporter gene was set in between P1 and HC-Pro gene. Fourth, they performed in vitro transcription-translation experiments. Products were labeled for detection. The results of this experiment showed that some HC-Pro point mutations lost their pathogenic activities while other did not. Four clones with HC-Pro cleavages showed reduced infectivity. In order to show that the reduction in infectivity was due to the missing HC-Pro gene, the researchers replaced the cleavage genes with the authentic HC-Pro and the clone infectivity was restored. Furthermore, some mutants still maintained their establishment of primary infection. These mutants were considered more competent than the other mutants that lost their establishment. In addition, all the mutants retained their HC-Pro proteinase activity. The maintenance of proteinase was not related to the ability of WSMV to establish primary infection. From the results, the researchers concluded that deletion of HC-Pro did not affect WSMV's pathogenicity because it still maintained protease activity. The result was consistent with the previous assumption of WSMV's pathogenicity.[17]
"cDNA cloning and nucleotide sequence of the wheat streak mosaic virus capsid protein gene" by C.L. Niblett, K.R.Zagula, L.A. Calvert, T.L. Kendall, D.M. Stark, C.E. Smith, R.N. Beachy and S.A. Lommel
The wheat streak mosaic virus looked very similar to the potyviruses. Both of them also had a cylindrical inclusion protein about the same size at 66K. However, the WSMV was transferred to the crop by the wheat curl mite vector. In contrast, the potyvirus was transferred by the aphid vector. From study, the capsid protein of WSMV was about 45K. It was larger than the capsid protein of potyviruses which range about 32K to 36K. In this study, the researchers aimed to perform cDNA cloning, in vitro transcription and translation, and the encoding capsid protein gene of WSMV. The gene sequence of WSMV's capsid proteins from the study were then compared with the gene sequence of the fungus-transmitted virus and aphid-transmitted virus. The method of the study involved four steps. In the first step, the researchers first purified the WSMV and isolated its RNA genome. They first inoculated the WSMV with corn seedlings and extracted the RNA with organic solvents. RNA was purified by cellulose affinity chromatography. In the next step, they performed the cDNA synthesis and cloning. The first and second strand of RNA were synthesized separately. The double stranded cDNA were then introduced to E.Coli. The third step was DNA sequencing using Sanger dideoxy method. In the last step, the in vitro RNA transcription, translation, immunoprecipitation and protein sequencing were carried out. The recombinant plasmid was chosen to translate and immunoprecipitate. Buffer was used for the products' suspension. At last, the protein sequence was determined by using Applied Biosystems Model 470A. From interpretation of the results, the translation products did not match in size with the capsid protein. Comparing the nucleotide sequence of WSMV and aphid-transmitted potyviruses, they had only 22 to 25% similarity. The nucleotide sequence of WSMV and the fungus-transmitted virus had only 12% identity. The researchers came to the conclusion that it was insufficient if they just used the capsid protein gene comparison. Despite of the many similarities of cylindrical inclusion proteins in both WSMV and potyvirus group, the capsid protein gene did not prove that there were sufficient similarities for the capsid protein gene.[18]
"Field Evaluation of Transgenic and Classical Sources of Wheat Streak Mosaic Virus Resistance" by G.L Sharp, J.M. Martin, S.P. Lanning, N.K. Blake, C.W. Brey, E. Sivamani, R.Qu, and L.E. Talbert
This research was the evaluation of the field studies in 1999 and 2000. Scientists tried to create a wheat type that was resistant to the infection of WSMV. There were many attempts before for this effort. One of the approaches was to incorporate viral transgenes into the wheat using the NIb and CP gene of WSMV. However, the result showed that the resistance was postponed. Another approach was conducted by using the cultivars that had more resistance than others. The aim of this study was to compare the resistance of the transgenic wheat and the cultivars from wheat Thinopyrum germplasm. Conditions of the field might be different than the green house conditions that previous studies had shown. Then from the results of evaluation, the researchers wanted to see the differences in the level of resistance. If possible, recommendations might be referred to control the infection of WSMV. In this experiment, the field trials took place at the Arthur H.Post Field Research Farm. The planting dates were 12 May 1999 and 3 May 2000. The seeds of cultivars were planted randomized in each row. Carborundum was also used to help viral transmission. The transgenic lines had their DNA extract, tagged site primers and sequencing. These described techniques were already used by the other studies. The infection symptoms were rated by Shahwan and Hill's method on the scale of 0-5. The presence of the virus was detected by using PAS-ELISA. The flour and grain proteins from trangenic wheat and cultivars were also collected for evaluation. The results of the study came out significantly for both types. The cultivars developed infection symptoms with high level of viral accumulation in the leaves. Resistance to WSMV was low. Yield reduction ranged from 51% to 77%. Similarly, the transgenic wheat were also infected. There was also high level of viral concentration. Yield reduction ranged from 46% to 47%. So the transgenic wheat was also not much better at WSMV resistance than the cultivars as the results from greenhouse trials in the other studies had shown. The researchers claimed maybe this was due to the different conditions during cultivation in the greenhouse trials and the field. There were no significant that showed a single type of wheat had a great resistance to WSMV.[19]